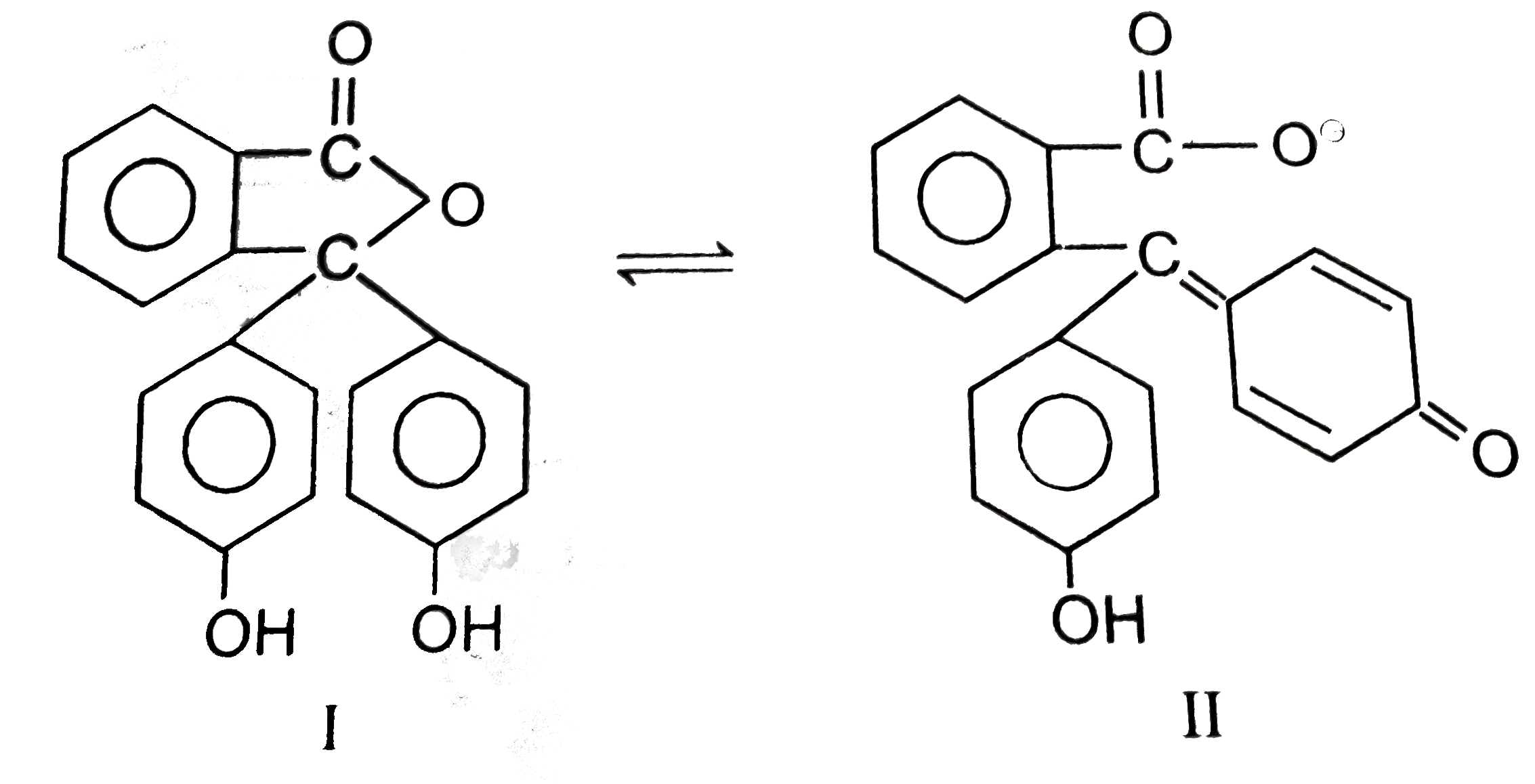
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
GRB PUBLICATION-IONIC EQUILIBRIUM-All Questions
- During the neutralisation of an acid by a base, the end point refers t...
Text Solution
|
- During the neutralisation of an acid by a base, the end point refers t...
Text Solution
|
- During the neutralisation of an acid by a base, the end point refers t...
Text Solution
|
- During the neutralisation of an acid by a base, the end point refers t...
Text Solution
|
- The Ph of basic buffer mixtures is given by : Ph=Pk(a)+log (["Base"])...
Text Solution
|
- The Ph of basic buffer mixtures is given by : Ph=Pk(a)+log (["Base"])...
Text Solution
|
- The Ph of basic buffer mixtures is given by : Ph=Pk(a)+log (["Base"])...
Text Solution
|
- The Ph of basic buffer mixtures is given by : Ph=Pk(a)+log (["Base"])...
Text Solution
|
- The solubility product of a soluble salt A(x)B(y) is given by : K(sp)=...
Text Solution
|
- The solubility product of a soluble salt A(x)B(y) is given by : K(sp)=...
Text Solution
|
- The solubility product of a soluble salt A(x)B(y) is given by : K(sp)=...
Text Solution
|
- The solubility product of a soluble salt A(x)B(y) is given by : K(sp)=...
Text Solution
|
- The solubility product of a soluble salt A(x)B(y) is given by : K(sp)=...
Text Solution
|
- For the self-ionisation (protonation) process liquid formic acid at 20...
Text Solution
|
- For the self-ionisation (protonation) process liquid formic acid at 20...
Text Solution
|
- For the self-ionisation (protonation) process liquid formic acid at 20...
Text Solution
|
- Amino acid glycine (NH(2)-CH(2)-COOH) exists as a zwitter ion in aq. S...
Text Solution
|
- Amino acid glycine (NH(2)-CH(2)-COOH) exists as a zwitter ion in aq. S...
Text Solution
|
- Following titration method is taken to compute stepwise ionisation con...
Text Solution
|
- Following titration method is taken to compute stepwise ionisation con...
Text Solution
|