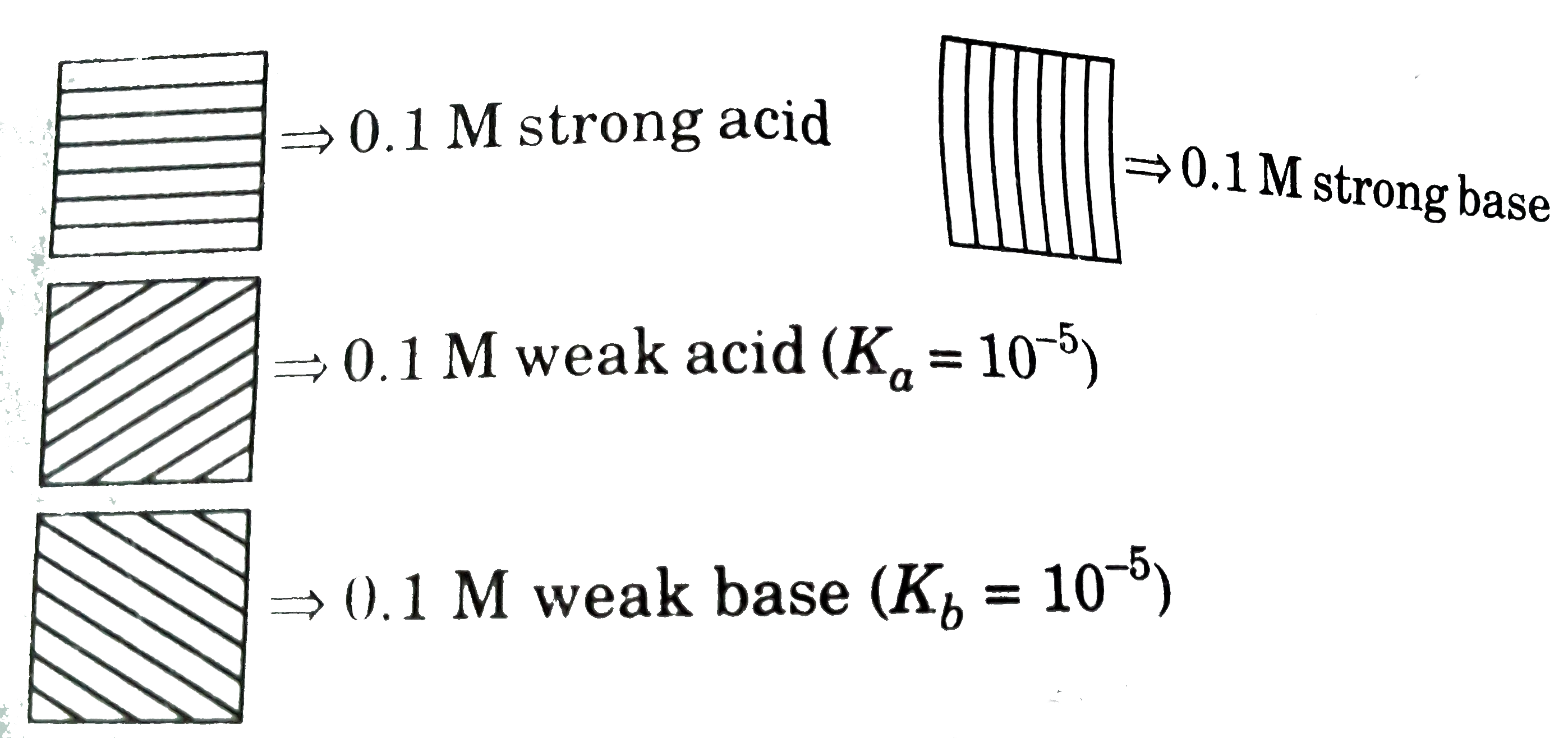A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-IONIC EQUILIBRIUM-All Questions
- Human blood has a narrow Ph range of 7.3-7.4, which must be maintained...
Text Solution
|
- Human blood has a narrow Ph range of 7.3-7.4, which must be maintained...
Text Solution
|
- We represent various reagents pictorially such that, The relative...
Text Solution
|
- 200 ml of an aqueous solution contains 0.1 M NaOH and 0.2M NH(4)OH(aq)...
Text Solution
|
- 200 ml of an aqueous solution contains 0.1 M NaOH and 0.2M NH(4)OH(aq)...
Text Solution
|
- Successive dissociation constants of H(2)CO(3) are K(a(1)) = 10^(-3) a...
Text Solution
|
- Successive dissociation constants of H(2)CO(3) are K(a(1)) = 10^(-3) a...
Text Solution
|
- 1 litre of 1M CH(3)COOH (very weak acid) taken in a container initiall...
Text Solution
|
- 1 litre of 1M CH(3)COOH (very weak acid) taken in a container initiall...
Text Solution
|
- 1 litre of 1M CH(3)COOH (very weak acid) taken in a container initiall...
Text Solution
|
- Refer to an aqueous solution of formic acid, HCOOH, which has a K(a) v...
Text Solution
|
- Refer to an aqueous solution of formic acid, HCOOH, which has a K(a) v...
Text Solution
|
- Match the effect of addition of 1M NaOH to 50 mL of 1M H(2)C(2)OH (dip...
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- How much water (in ml) must be added to 300 mL of a 0.2 M solution of ...
Text Solution
|
- In the titration of a solution of a weak base BOH with HCI has pH is 8...
Text Solution
|
- The acidic strength of two monobasic acids may be compared by comparin...
Text Solution
|
- The dissociation constant of a substituted benzoic acid at 25^(@)C is ...
Text Solution
|
- Amongst the following, the total number of compounds whose aqueous sol...
Text Solution
|