Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-SURFACE CHEMISTRY-SUBJECTIVE TYPE
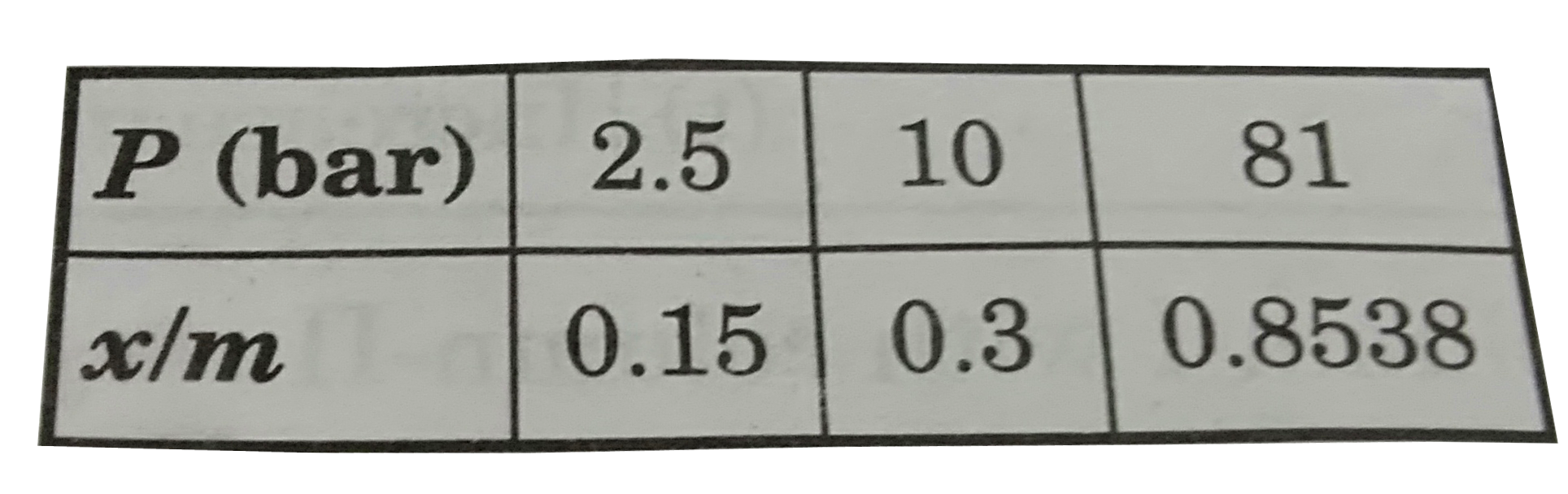
- In a particular case of physisorption, magnitude of enthalpy change an...
Text Solution
|
- Molecular formula of starch can be represented as (C(6)H(10)O(5))(n). ...
Text Solution
|
- On addition of 1 mL solution of 10% NaCl to 10 mL gold sol in the pres...
Text Solution
|
- Calculate the number of sols which are negatively charged.
Text Solution
|
- Calculate coagulation value of AlCl(3) if for coagulation of 400 ml of...
Text Solution
|
- At a temperature of 180 K, O(2) gas adsorbs on Pt surface following Fr...
Text Solution
|
- For the coagulation of 100 mL of arsenious sulphite sol,5 mL of 1 M Na...
Text Solution
|
- In order to cause coagulation of 200 ml of gold sol, 585 ml 1% w/w NaC...
Text Solution
|
- 6.84 gmAl(2)(SO(4))(3) is needed to coagulate 2.5 L of As(2)S(3) sol c...
Text Solution
|
- A container contains 1 litre, 2 M solution of cyclobutane in either. A...
Text Solution
|
- In a vessel O(2) gas molecules were adsorbed on solid surface causing ...
Text Solution
|
- Count the number of correct statements. (a) Minimum potential requir...
Text Solution
|
- How many of the following may cause coagulation in colloidal solutions...
Text Solution
|