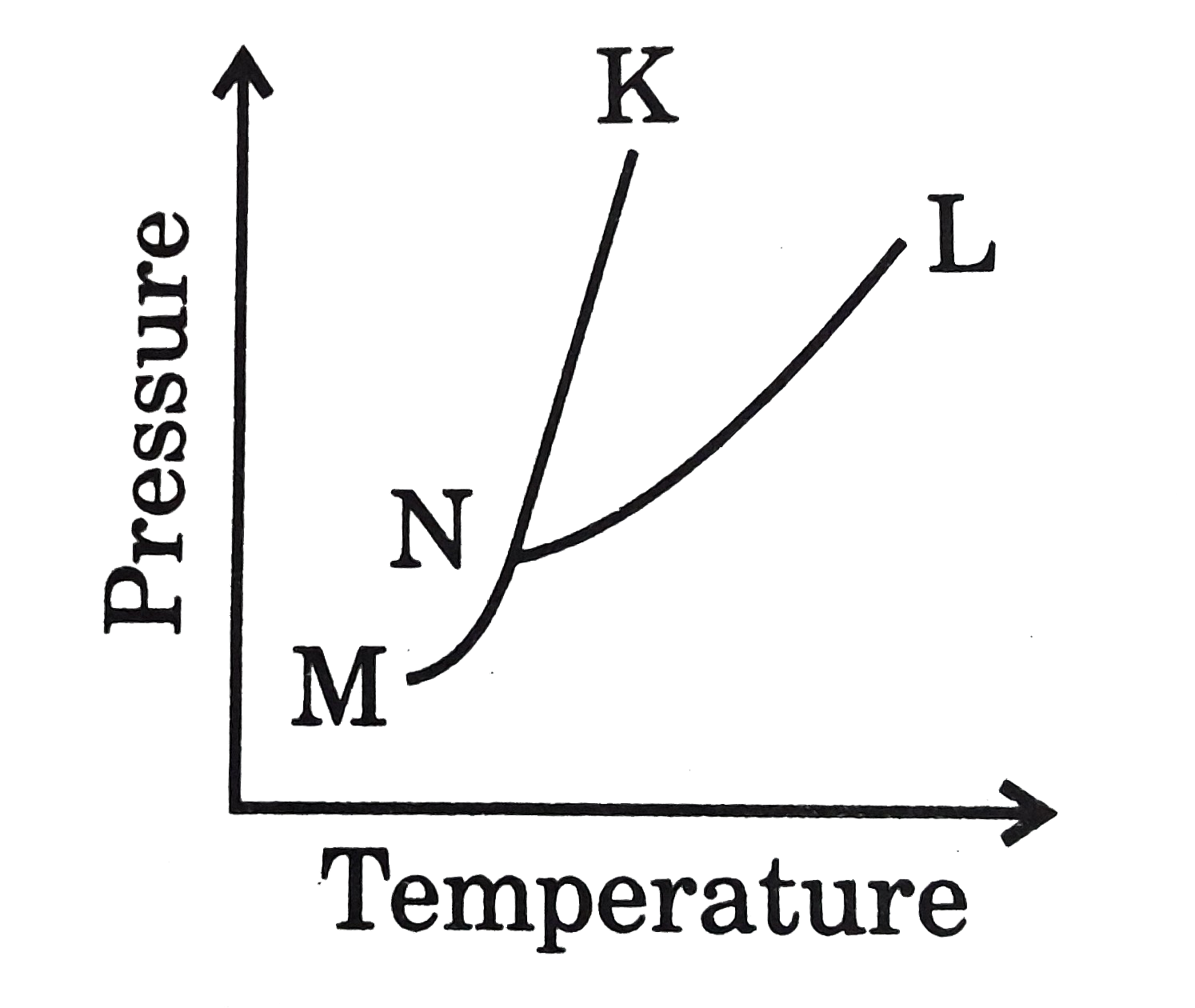Similar Questions
Explore conceptually related problems
Recommended Questions
- According to the phase diagram shown, where does a mixture of solid an...
Text Solution
|
- SOLID-LIQUID EQUILIBRIUM, LIQUID-VAPOUR EQUILIBRIUM AND SOLID-VAPOUR E...
Text Solution
|
- According to le-Chatelier's principle, adding heat to a solid and liqu...
Text Solution
|
- The concentration of a pure solid or liquid phase is not include in th...
Text Solution
|
- According to the phase diagram shown, in what state does not represent...
Text Solution
|
- According to this phase diagram, which phases can exist at pressure lo...
Text Solution
|
- According to the phase diagram shown, where does a mixture of solid an...
Text Solution
|
- At a particular temperature and atmospheric pressure, the solid and li...
Text Solution
|
- At a particular temperature and atmospheric pressure, the solid and li...
Text Solution
|