Similar Questions
Explore conceptually related problems
Recommended Questions
- Assuming the formation of an ideal solution, determine the boiling poi...
Text Solution
|
- A solution of sucrose (molar mass =342) is prepared by dissolving 688....
Text Solution
|
- Assuming the formation of an ideal solution, determine the boiling poi...
Text Solution
|
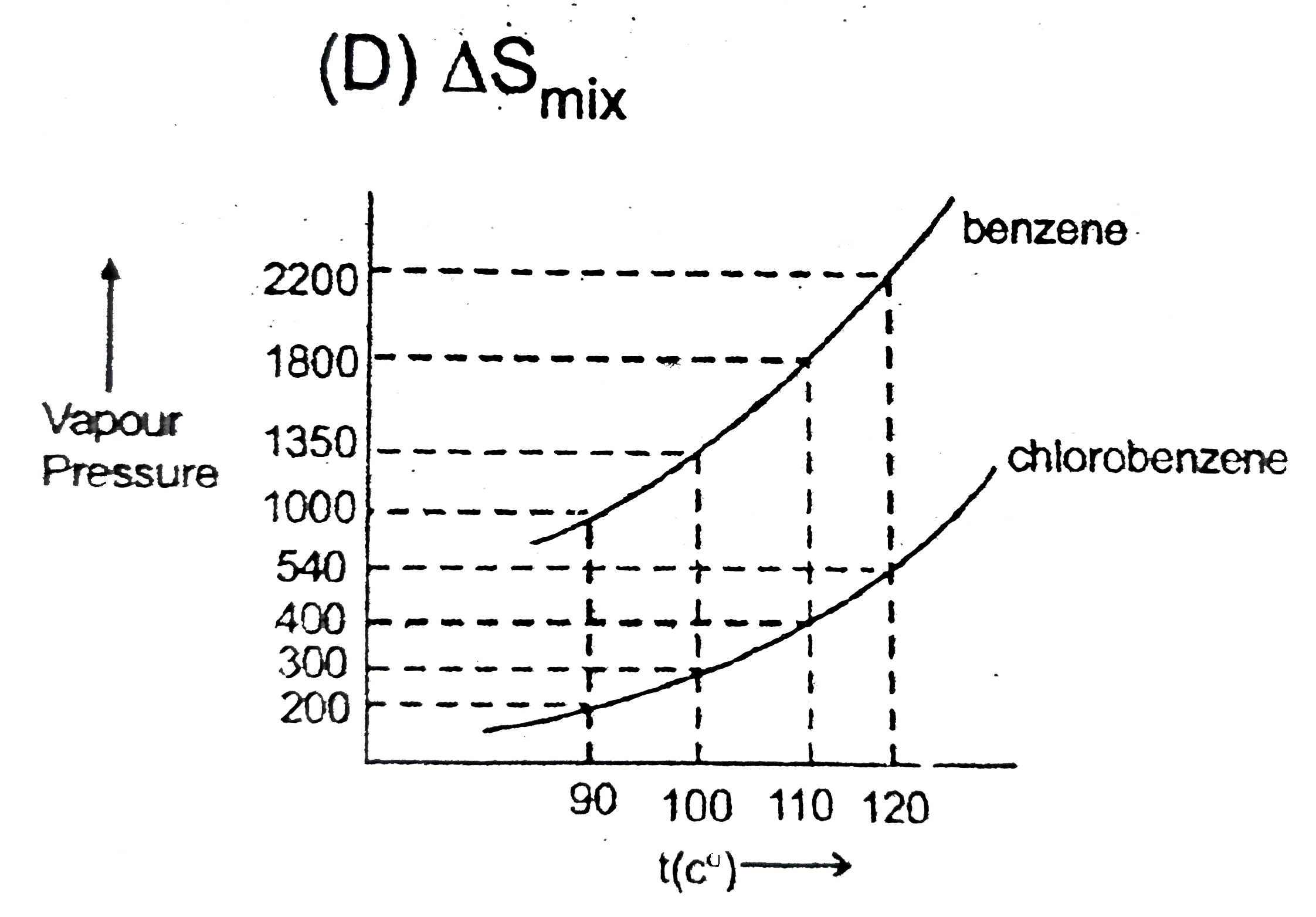
- The following is a table of the vapour pressure of pure benzene and ch...
Text Solution
|
- Benzene and toluene form nearly ideal solutions. At 20^(@) C, the vapo...
Text Solution
|
- The following is a table of the vapour pressure of pure benzene and ch...
Text Solution
|
- Assuming the formation of an ideal solution, determine the boiling poi...
Text Solution
|
- Define boiling point. Write the formula to determine molar mass of a s...
Text Solution
|
- 100g of liquid A(molar mass 140"g mol"^(-1)) was dissolved in 1000g of...
Text Solution
|