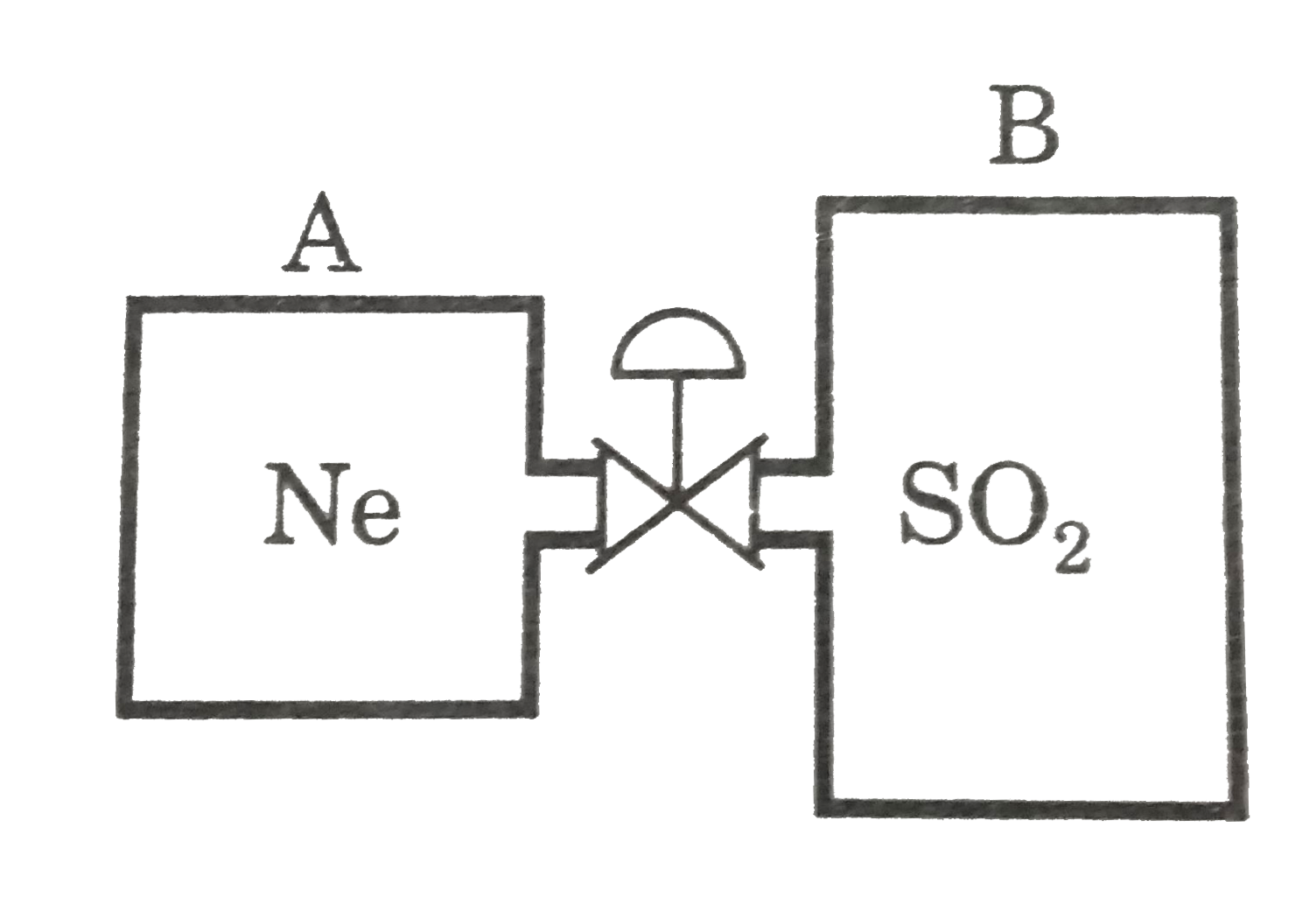
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-THERMODYNAMICS-All Questions
- What is the change internal energy when a gas contracts from 377 mL ...
Text Solution
|
- The heat capactiy of liquid water is 75.6 J//mol K, while the enthalpy...
Text Solution
|
- Two rigid adiabatic vessel A and B which initially ,contain two gases...
Text Solution
|
- Warming ammonium chloride with sodium hydroxide in a test tube is an e...
Text Solution
|
- Out of boiling point (P), entropy (Q), pH (R ) and e.m.f. of cell (S...
Text Solution
|
- Ice-water mass ratio is maintntained as 1:1 in a given system conta...
Text Solution
|
- A piece of zinc at a tempreature of 20.0^(@) C weighing 63.38 g is ...
Text Solution
|
- Two mole of an ideal gas is heated at constant pressure of one atmosp...
Text Solution
|
- Determine which of the following reactions at constant pressure rep...
Text Solution
|
- A sample of liquid in a thermally insulated constant ( a calorimetre...
Text Solution
|
- 10 mole of ideal gas expand isothermally and reversibly from a pressur...
Text Solution
|
- A gas expands adiabatically at constant pressure such that TpropV^(-...
Text Solution
|
- Which has maximum internal energy at 290K ?
Text Solution
|
- 100mL of a liquid is contained in an insulated container at a pressure...
Text Solution
|
- Consider the reaction at 300 K H(2)(g)+Cl(2)(g)to2HCl(g), DeltaH...
Text Solution
|
- For the real gases reaction, 2CO(g)+O(2)(g)to2CO(2)(g),DeltaH=-560 k...
Text Solution
|
- Correct statements about samples of ice and liquid water at 0^(@) C in...
Text Solution
|
- A heating coil is immersed in a 100 g sample of H(2)O (l) at a 1 atm ...
Text Solution
|
- 10 itres of monoatomic gas at 0^(@) C and 10 atm pressure is suddenly ...
Text Solution
|
- Consider a classroom that is roughly 5mxx10mxx3m. Initially T=27^(@)C ...
Text Solution
|