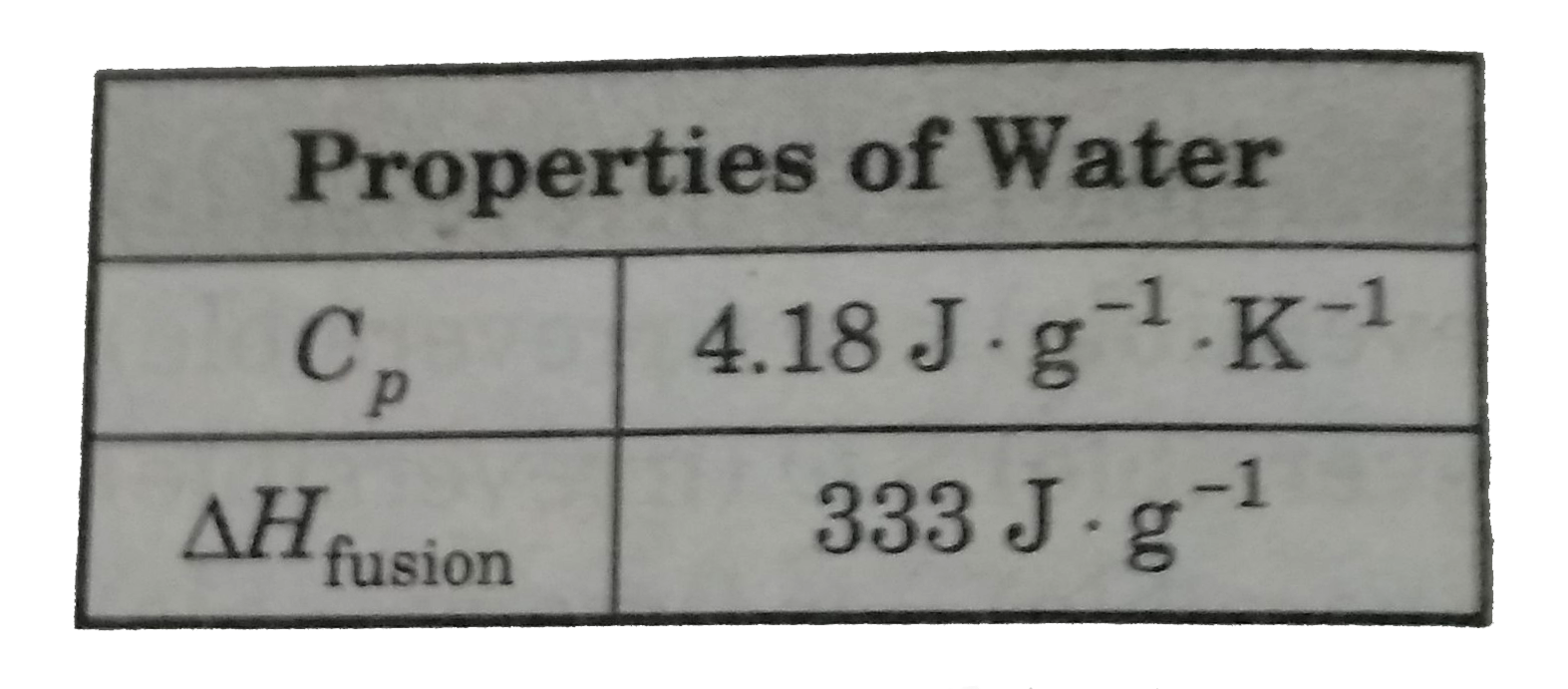
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-THERMODYNAMICS-All Questions
- A gold ring that weighting 3.81g is heated to 84^(@)Cand placed in 50....
Text Solution
|
- For which exothermic reaction is deltaE more negative than deltaH?
Text Solution
|
- An ice cube of unknown mass at 0^(@)C is added to 265g of H(2)O at 25....
Text Solution
|
- What is the specific heat capacity of mercury ("in" Jxxg^(-1)xxC^(-1))...
Text Solution
|
- For reactions conducted at constant pressure, under that what conditio...
Text Solution
|
- 84.12g "of gold at" 120.1^(@)C. Is placed in 106.4g of H(2)Og at 21.4...
Text Solution
|
- A 10.00g piece of metal is heated to 80.00^(@)C and placed in 100.0g ...
Text Solution
|
- When MgO reacts with H(2)O at 25^(@)C and 1 atm, the volume change is ...
Text Solution
|
- An ice cube at an unknown temperature is added to 25.0g of liquid H(2)...
Text Solution
|
- Which is (are) state properties? (P) enthalpy (Q) heat (C ) Volume
Text Solution
|
- How much work is done by the gas when 1.00g of sodium azide, NaN(3) (M...
Text Solution
|
- The specific heat capacities of three metals are given below. If...
Text Solution
|
- A system consists of a gas contined in a thin ballon. If the ballon de...
Text Solution
|
- Which of the following is a mathametical statement of the first law of...
Text Solution
|
- 7 g of N(2)g at 27^(@)C is expanded slowly and isothermally form press...
Text Solution
|
- An ideal gas involved in a reversible adiabatic process follow the law...
Text Solution
|
- One mole of H(2) at 25^(@)C undergo combustion in the presence of exce...
Text Solution
|
- 2(Ag)toB(g), deltaH=-20kcal//mol. In a closed rigid container, 0.2mole...
Text Solution
|
- A real gas is subjective to an adiabatic process form (2"bar",40L,300K...
Text Solution
|
- Calculate magnitude of work involved when 100g of calcite form of CaCO...
Text Solution
|