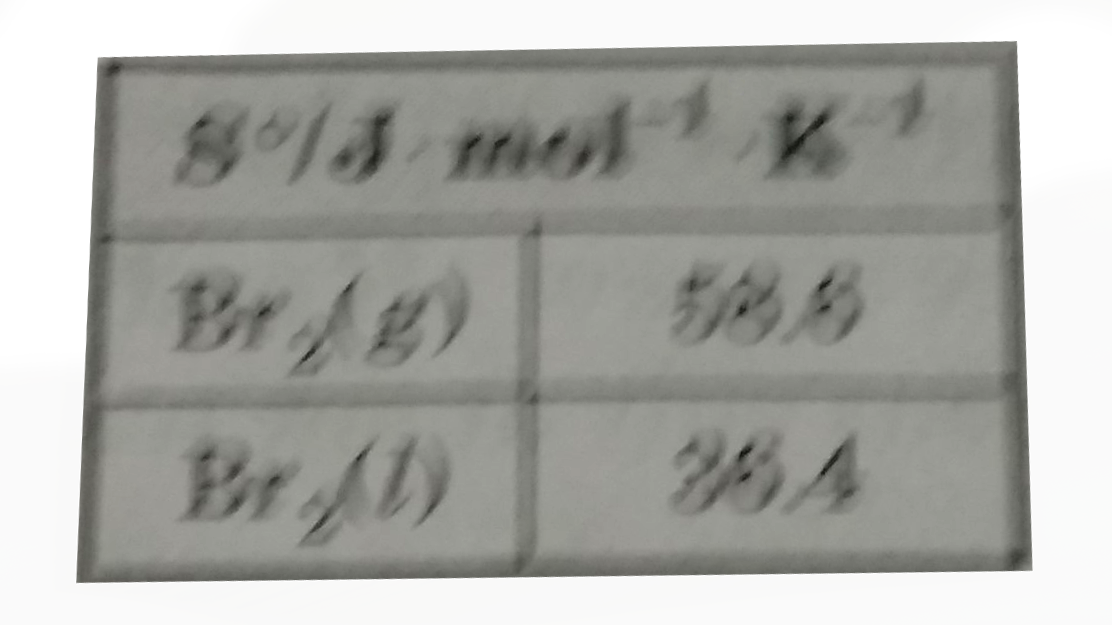A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-THERMODYNAMICS-All Questions
- For a reaction at 25%(@)C, DeltaG^(@)=-33.3kJ and DeltaS= -198kJ xxK^(...
Text Solution
|
- Which statement is always true for a spontaneous reaction?
Text Solution
|
- Liquid bromine boils at 332.7.K Estimate the enthalpy of formation of ...
Text Solution
|
- Which change(s) is(are) accompanied by an increase in entropy of the s...
Text Solution
|
- Which choice represents the signes for DeltaS and DeltaH for the subli...
Text Solution
|
- A reaction has DeltaH^(@) gt0 and DeltaG^(@) gt0 25^(@)C. This reacti...
Text Solution
|
- An ionic compound ha a solublity increases as the temperature is raise...
Text Solution
|
- The enthalpy of fusion for NaF(s) at its melting point (992^(@)C) is 2...
Text Solution
|
- For the reaction at 25^(@)C, C(2)H4(g)+H(2)(g) LeftrightarrowC(2)H(6...
Text Solution
|
- Which reaction has the most positive entropy change understandard cond...
Text Solution
|
- What are the sign of DeltaH and DeltaS for a reaction that is spontane...
Text Solution
|
- For a reaction at constant pressure to be spontaneous, which relations...
Text Solution
|
- Tungsten is obtained commercially by the reduction of WO(3) " with " H...
Text Solution
|
- The gases compound NOBr decomposses accordings to the equation NOBr(...
Text Solution
|
- At the triple point of water how do the entropies of solid, liquid, an...
Text Solution
|
- "A perfect crystalline substance has an entropy of zero at absolute ze...
Text Solution
|
- Which of the following sets of conditions would result in a reaction t...
Text Solution
|
- Spontaneous reactions always:
Text Solution
|
- Which is a statement of the second law of Thermodynamics?
Text Solution
|
- For the reaction, 2H(g) to H(2)(g), what are the signs of DeltaH and D...
Text Solution
|