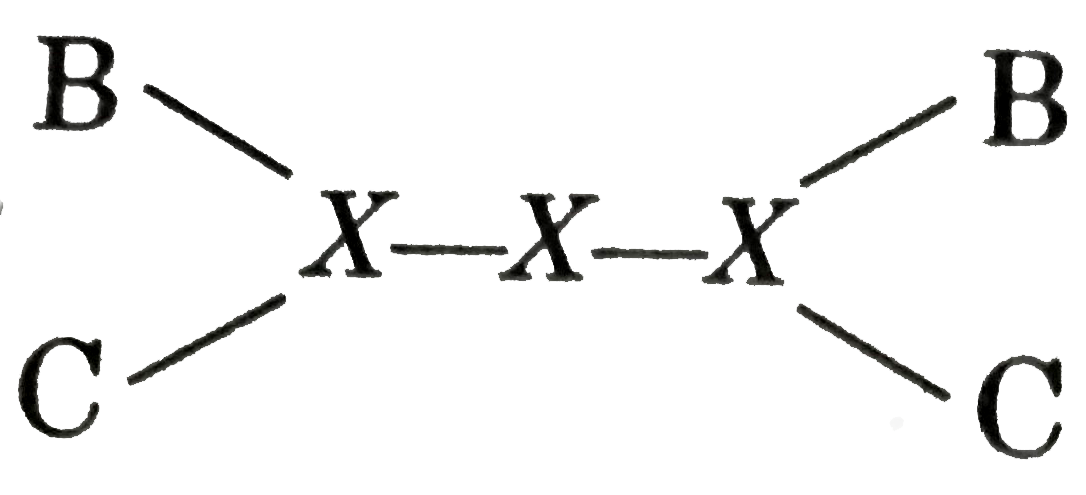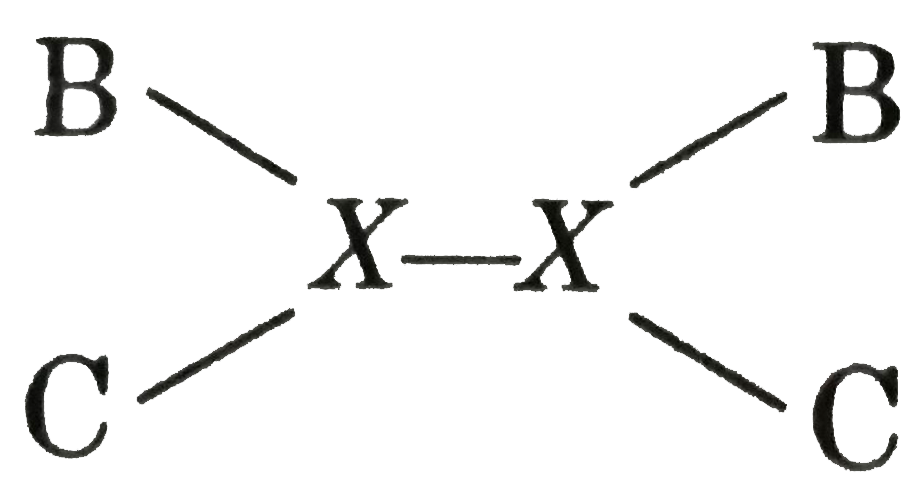A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-THERMODYNAMICS-All Questions
- Give the value of resonance energy for using these data :
Text Solution
|
- Calculate C-H bond energy from the following data : Delta(f)H" "[C...
Text Solution
|
- Calculate bond energy of X-X bond from the following data. DeltaH...
Text Solution
|
- The combustion of 1.22 g benzoic acid (M=122) in a bomb calorimeter at...
Text Solution
|
- It is observed that an combustion of 5.6 g of but-1ene(g) 70 kcal of h...
Text Solution
|
- It is observed that on combustion of 4.2 g of gaseous propene in a clo...
Text Solution
|
- Standard enthalpy of formation of N(2)O(5) is -100 kcal//mol and stand...
Text Solution
|
- The bond energy of C=O if DeltaH(f)^(@)(CO(2))=390 kJ, DeltaH("sublima...
Text Solution
|
- What will be the maximum amount of heat realeased when 321 g of a mixu...
Text Solution
|
- Which of the followig substances willl have non-zero standard enthalpy...
Text Solution
|
- What will be the value of resonance energy of N(2)O if : DeltaH(BDE)...
Text Solution
|
- For the reaction, 2NH(3)(g)toN(2)(g)+3H(2)(g) Identify the statement...
Text Solution
|
- Identify the option which is correct :
Text Solution
|
- Calculate DeltaH when 2 moles of solid benzoic acid undergo complete c...
Text Solution
|
- Two solids A and B having molar masses 200 and 300 react to form anoth...
Text Solution
|
- A(g)to2B(g),DeltaH^(@)=10 kJ//mole at 300 K, C(P,A)=20 J//Kmole ...
Text Solution
|
- Calculate DeltaH("combustion"^(@) of C("graphite") if DeltaH(f)^(@) of...
Text Solution
|
- The bond enthalpies of C-C,C=C and C=C bonds are 348, 610 and 835 kj//...
Text Solution
|
- Given the following data : Determine at what temperature the foll...
Text Solution
|
- If DeltaH(f)^(@) for Ag^(+) (infinately diluted), NO(3)^(-) (infinity ...
Text Solution
|