A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-THERMODYNAMICS-All Questions
- Calculate DeltaH("combustion"^(@) of C("graphite") if DeltaH(f)^(@) of...
Text Solution
|
- The bond enthalpies of C-C,C=C and C=C bonds are 348, 610 and 835 kj//...
Text Solution
|
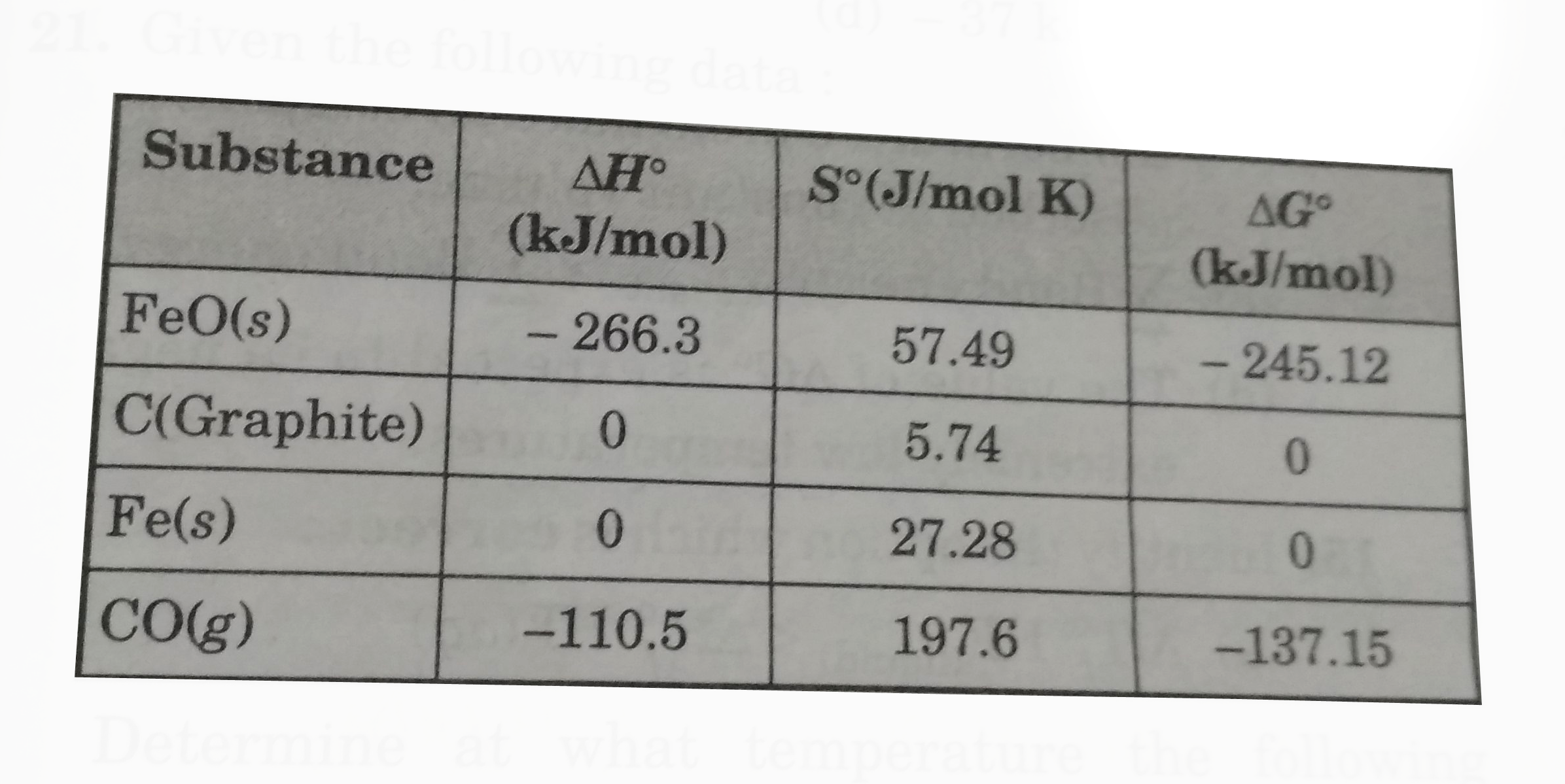
- Given the following data : Determine at what temperature the foll...
Text Solution
|
- If DeltaH(f)^(@) for Ag^(+) (infinately diluted), NO(3)^(-) (infinity ...
Text Solution
|
- IN Haber's process of manufacturing of ammonia : N(2)(g)+3H(2)(g)to2...
Text Solution
|
- Which of the reaction defines molar DeltaH(f)^(@) ?
Text Solution
|
- In the reaction, CO(2)(g)=H(2)(g)toCO(g)=H(2)O(g)," "DeltaH=2.8 ...
Text Solution
|
- Given, H(2)(g)+Br(2)(g)to2HBr(g),DeltaH(1)^(@) and standard enthal...
Text Solution
|
- For the following reaction, C("diamond")+O(2)toCO(2)(g), DeltaH=-94....
Text Solution
|
- C(s)+O(2)(g)toCO(2)(g)," "DeltaH=-94.3 kcal//mol CO(g)+(1)/(2)O(2...
Text Solution
|
- The difference between DeltaH and DeltaE on a molar basis for the comb...
Text Solution
|
- The standard heat of combustion of solid boron is equal to :
Text Solution
|
- From the following data of DeltaH, of the following reactions C(s)+(...
Text Solution
|
- 2 mole of zinc is dissolved in HCl at 25^(@) C. The work done in open ...
Text Solution
|
- A solution of 500mL of 2M KOH is added to 500mL of 2M HCl and the mixt...
Text Solution
|
- One mole of anahydrous MgCl(2) dissolves in water and liberates 25 cal...
Text Solution
|
- C(2)H(6)(g)+3.5O(2)(g)to2CO(2)(g)+3H(2)O(g) DeltaS("vap")(H(2)O,l)=x...
Text Solution
|
- Consider the DeltaG(f)^(@) and DeltaH(f)^(@) (kJ//mol) for the followi...
Text Solution
|
- If DeltaG=-177 kcal for " "(1)2Fe(s)+(3)/(2)O(2)t...
Text Solution
|
- The bond dissociation energy of gaseous H(2),Cl(2) and HCl are 104,58,...
Text Solution
|