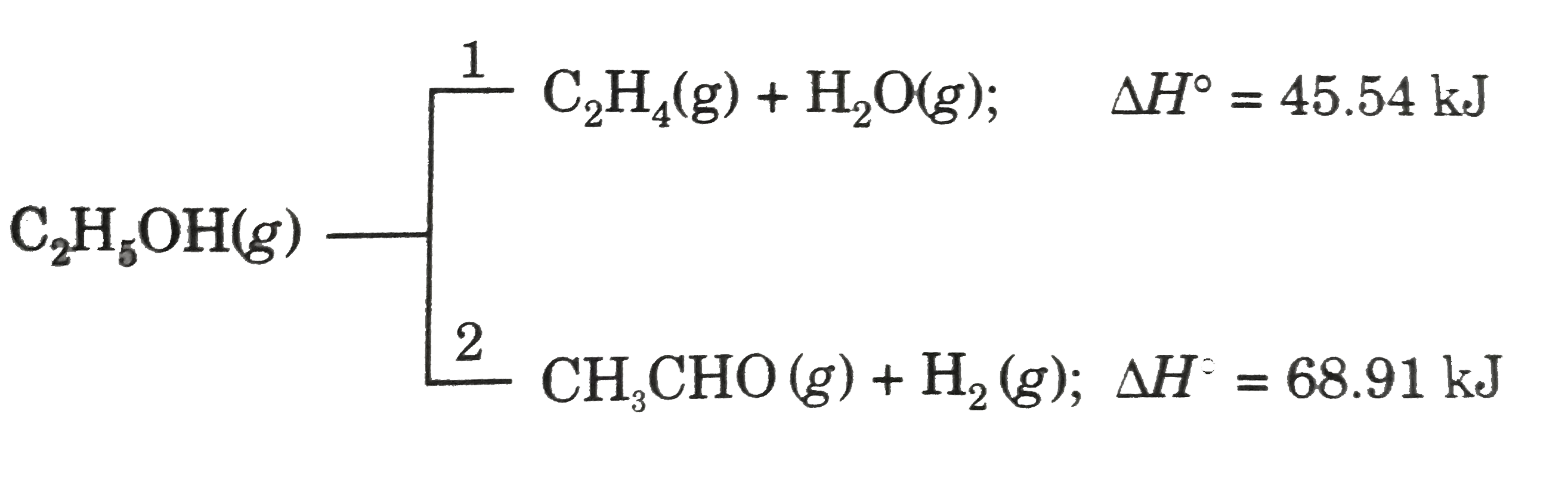A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-THERMODYNAMICS-All Questions
- The enthalpy of combustion of propance (C(3)H(8)) gas in temes of give...
Text Solution
|
- For the following reaction, {:(C("diamond")+O(2)to CO(2)(g),, DeltaH...
Text Solution
|
- Ethanol can undergo decompostion to form two sets of products. If th...
Text Solution
|
- (p) Cis-2- butene to trans -2-butene, DeltaH(1) (Q)Cis-2-buteneto 1-...
Text Solution
|
- Calculate the amount of heat released at constant pressure when 10 mol...
Text Solution
|
- The reaction CH(4)(g)+Cl(2)(g)to CH(3)Cl(g)+HCl(g) has DeltaH=-25kcal ...
Text Solution
|
- Calculate the ethanly change for the given reaction from data provided...
Text Solution
|
- Lattice energy of Na(2)CO(3) is -205 KJ/mole and hydrogen energy of Na...
Text Solution
|
- If x(1) , x(2) and x(3) are enthalpies of H-H , O=O and O-H bonds resp...
Text Solution
|
- NH(3)(g)+3Cl(2)toNCl(3)(g)+3HCl(g),DeltaH(1) N(2)(g)+3H(2)(g)to2NH(3...
Text Solution
|
- For the combustion of 1 mole of liquid benzene at 27^(@)C the heat of ...
Text Solution
|
- Consider the equation: 4PH(3)(g)+80(2)(g)toP(4)O(10)(s)+6H(2)O(g), ...
Text Solution
|
- Molar enthalpy of combustion of C(2)H(2)(g),C("graphite")andH(2)(g) ar...
Text Solution
|
- If CH(3)COOH+OH^(-)toCH(3)COO^(-)+H(2)O+q(1)and H^(+)+OH^(-)to H(2)O+q...
Text Solution
|
- The enthalpy of solution , sodium and sodium oxide in large volume of ...
Text Solution
|
- 0.2 M, 100ml NaOH is mixed wih 0.4 M, 100mL HCl solution . Determine ...
Text Solution
|
- The heat or rectin does not depend upon :
Text Solution
|
- Which of the following enthalpy may be positive or negative?
Text Solution
|
- Heat of reaction of C("diamond")+2S(s) to CS(2)(l) is known as :
Text Solution
|
- What is heat of submisation of P(4)O(6)(s)? Given heat of sublimatio...
Text Solution
|