A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-THERMODYNAMICS-All Questions
- For the given reaction: H(2)(g)+Cl(2)(g) to2H^(+)(aq)+2Cl^(-)(aq) ...
Text Solution
|
- Using the data provided, calculate the multiple bond energy (kJ mol^(-...
Text Solution
|
- Calculate the Enthalpy of hydrogenation of If the Delta(f)H of and a...
Text Solution
|
- The enthalpies of vombustion of formaldehyde and paraformaldehyde (a p...
Text Solution
|
- Glucose when dissolved in water leads to cooling of the solution . Sup...
Text Solution
|
- Consider this equation and the associated value for DeltaH^(@). 2H(2...
Text Solution
|
- A bomb calorimeter has a heat capacity of 783J xx^(@)c^(-1) and contai...
Text Solution
|
- Determine the heat of reaction for this process Fe(s)+O(2)(g)to2FeO(...
Text Solution
|
- Use bond energy to estimate DeltaH for this rection: H(2)(g)+O(2)(g)...
Text Solution
|
- For which of these is DeltaH(f)^(@) not equal to zone?
Text Solution
|
- The enthalpy change change for which reaction represents the standard ...
Text Solution
|
- What is the standed enthalpy of formation of MgO(s) is 300.9 Kj is evo...
Text Solution
|
- For the formation of one mole of each of these gases from their elemen...
Text Solution
|
- 4Li(s)+O(2)(g)to2Li(2)O(s) At 25^(@)C,DeltaH for this reaction is -5...
Text Solution
|
- The heat of formation of NO from its elements is +90KJmol^(-1) What is...
Text Solution
|
- Fe(2)O(2)(s)+(3)/(2)C(s)to(3)/(2)CO(2)(g)+2Fe(s) DeltaH^(@)=+234.12K...
Text Solution
|
- Which combination of solution of Hcl and NAOH would produce the larges...
Text Solution
|
- Consider this reaction . 2N(2)H(4)(l)to3N(2)(g)+4H(2)O(g) DeltaH=-10...
Text Solution
|
- Use the information in the table to calculate the enthalpy of this rea...
Text Solution
|
- 50.0mL of 0.10 M HCl is mixed with 50.0mL of 0.10 M NaOH .The solution...
Text Solution
|
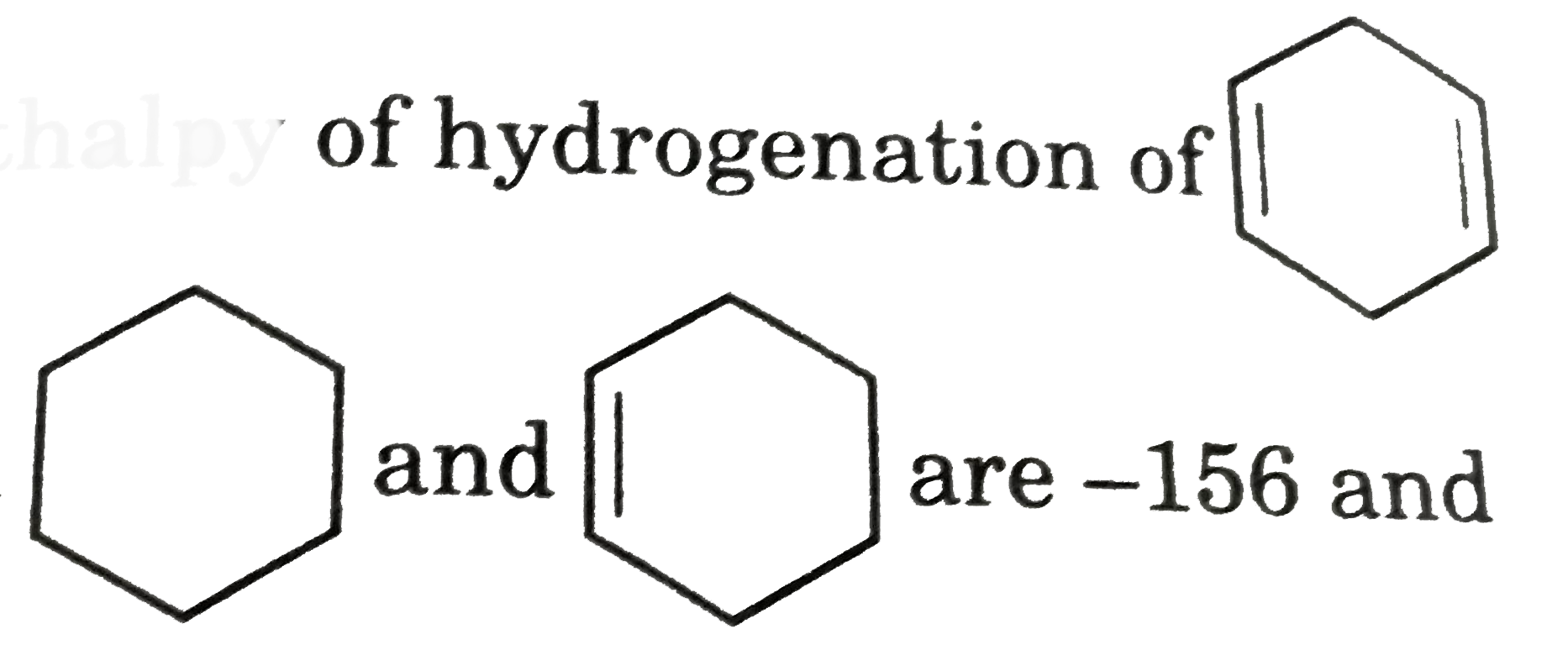 are -156 and -37 Kj /mol respectively.
are -156 and -37 Kj /mol respectively.