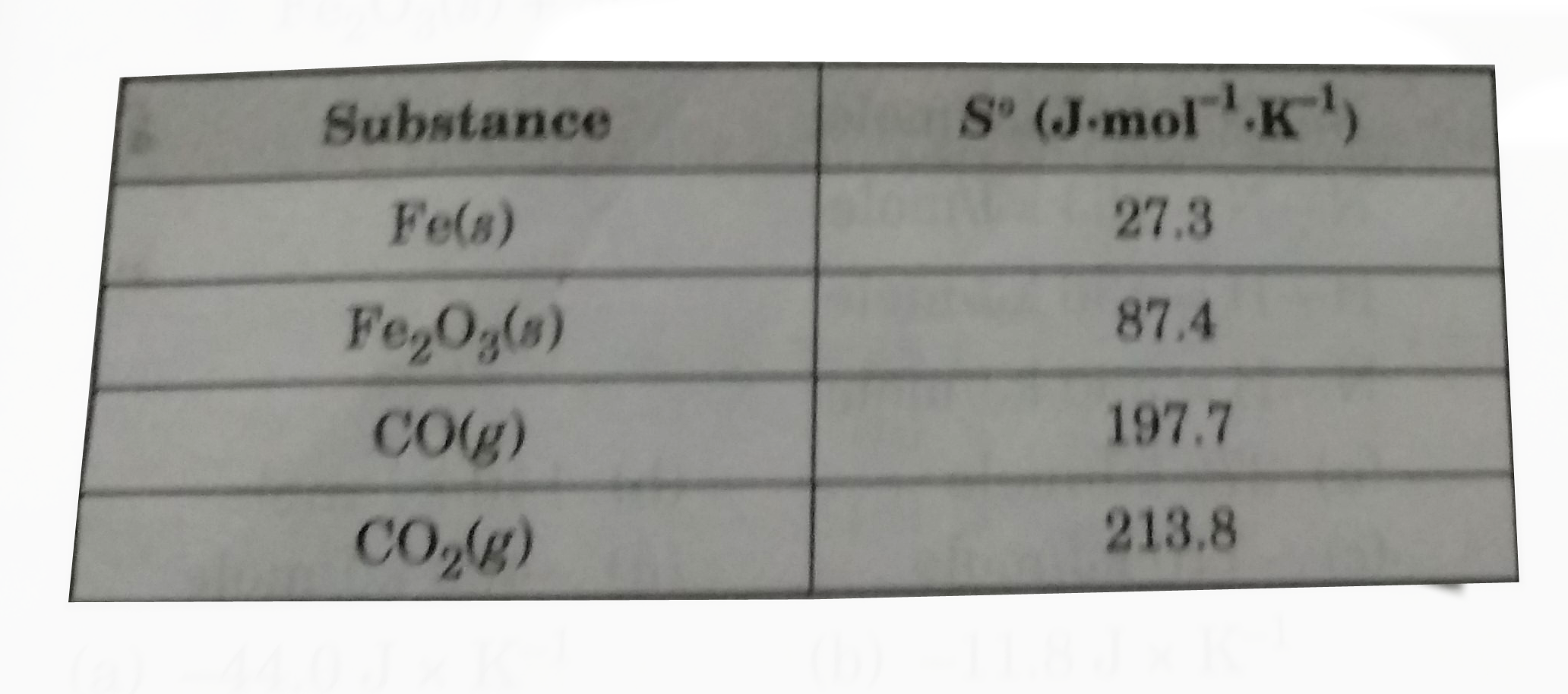A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-THERMODYNAMICS-All Questions
- Determine the enthalpy change for the reaction of 5.00 g Fe(2)O(3)with...
Text Solution
|
- The energies of the bonds broken in a certain eaction arae greater th...
Text Solution
|
- What is the value of Delta s^(@) for the reaction below? Fe(2)O(3)(...
Text Solution
|
- Statement-1: Adiabatic free expansion of any subatance in a cosed syst...
Text Solution
|
- Statement-1: The heat absorbed during the adiabatic expansion of an a ...
Text Solution
|
- Consider a reaction : A(g)+2b(g)toC(g) DeltaH(300)^(@)=40KJ, Delta...
Text Solution
|
- Statement-1: The enthalpy of formation of H(2)O(l)is greater than of H...
Text Solution
|
- Statement -1: heat of neutralistion of perchoric acid ,HClO(4) with Na...
Text Solution
|
- Statement -1: When a gas at high pressure expands against vaccum, the...
Text Solution
|
- Statement -1 in the following reaction : C(s)+O(2)(g)to CO(2) (g), D...
Text Solution
|
- Staement -1: The heat absorbed during the isothermal expansion of an i...
Text Solution
|
- Staetement -1: The magniyude of the work involed in an isothermal expa...
Text Solution
|
- Statement -1: Entropy change in reversible adiabatic expansion of an i...
Text Solution
|
- Statement -1: The Standard free energy changes of all spontaneously oc...
Text Solution
|
- Statement -1: Enthalpy and entropy of any elements substance in the st...
Text Solution
|
- Statement-1: A reaction which is spontaneous and accompained by decrea...
Text Solution
|
- Statement -1: Many endothermic reactions that are not spontaneous at r...
Text Solution
|
- Statement-1: Decrease of free energy during the process under constant...
Text Solution
|
- Statement -1: For every chmical reaction at equilibrium , standard Gid...
Text Solution
|
- Statement-1: There is a natural asymmetry between work to heat and con...
Text Solution
|