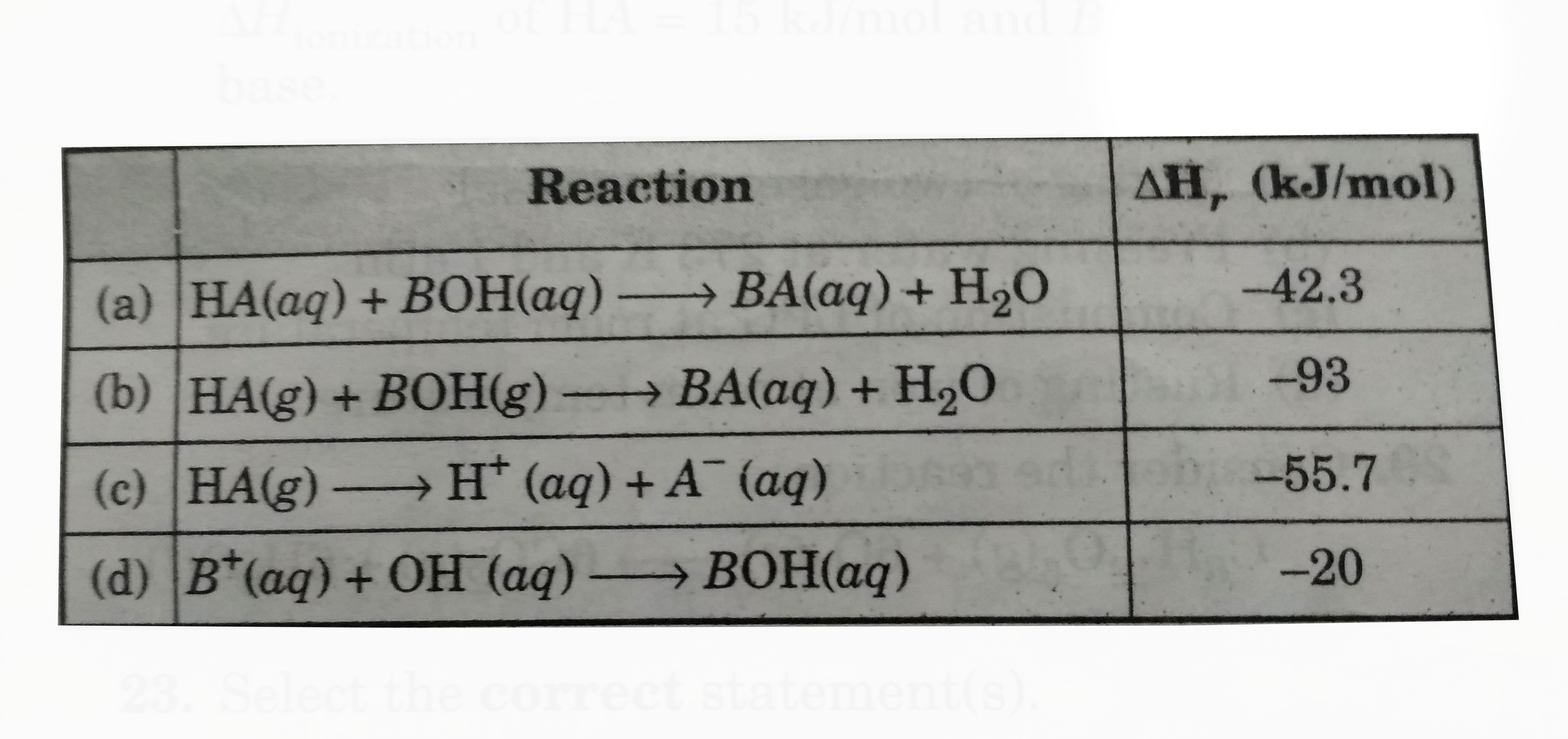
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-THERMODYNAMICS-All Questions
- Which of the following stament (s) is/are false ?
Text Solution
|
- Which of the following stament (s) is/are false ?
Text Solution
|
- From the following date , mark the opation(s) where Delta H is correct...
Text Solution
|
- Select the correct statement (s).
Text Solution
|
- Select the correct statement for an ideal gas undergoinf reversibl...
Text Solution
|
- For the charge : R to P: " "DeltaH=-ve If in above ...
Text Solution
|
- Which of the following statement is .are correct ?
Text Solution
|
- For a soild A(s) following sate is giiven is given DeltaH (fusion...
Text Solution
|
- Which of the following processe will lead to increase in entropy of u...
Text Solution
|
- Consider the reaction, C(6)H(12)O(6)(g)+6O(2)(g) to 6CO(2)(g) +6H(2...
Text Solution
|
- The integral enthaiply of solution of one mole of H(2)SO(4) with n mo...
Text Solution
|
- The standard enthaply of fromation of CO(2)will be given by:
Text Solution
|
- Which of the following is always negative ?
Text Solution
|
- Which is an irreversible processe ?
Text Solution
|
- Select the correct option :
Text Solution
|
- The correct statement (s) is/are:
Text Solution
|
- Which of the following statement is /are correct ?
Text Solution
|
- Select the correct option(s).
Text Solution
|
- Select the incorrect statement (s) .
Text Solution
|
- Thermodynamics mainly deals with:
Text Solution
|