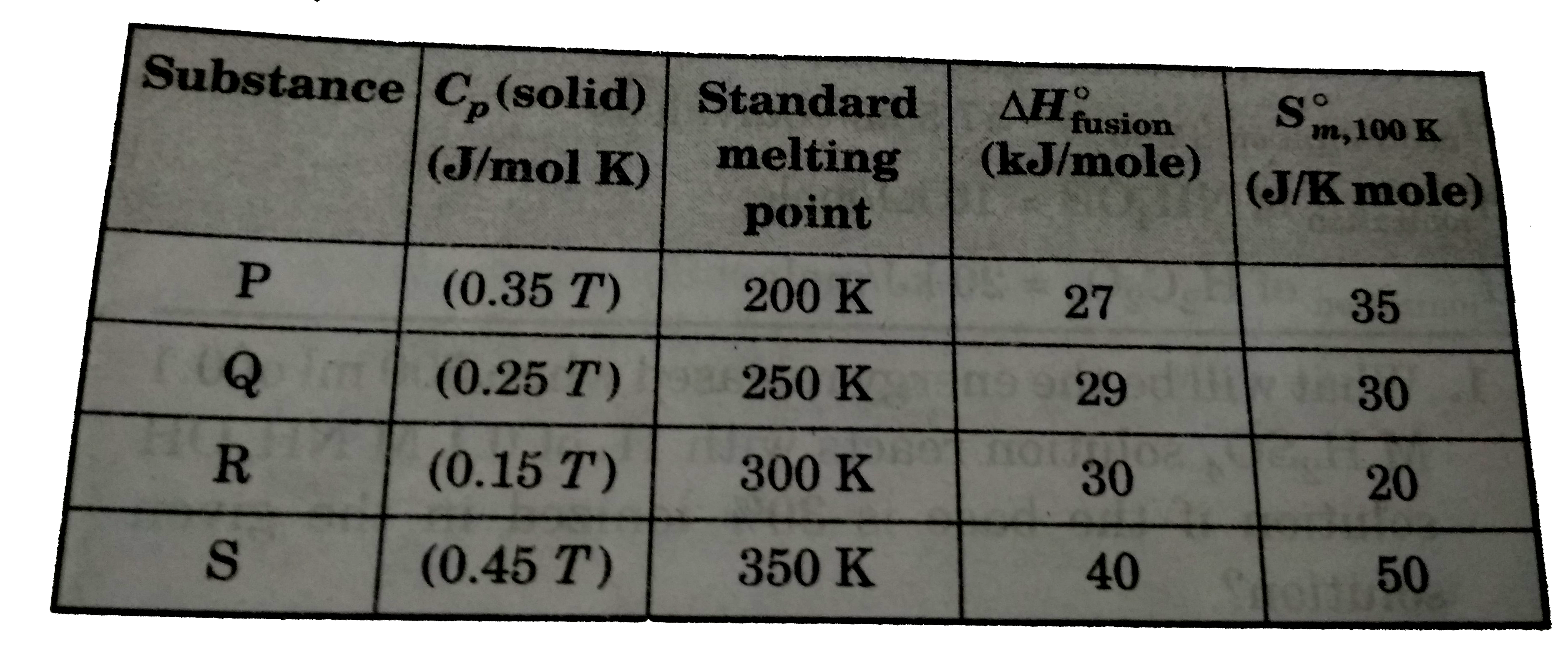
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-THERMODYNAMICS-All Questions
- Bomb calorimeters are the devices that are used to experimentally ...
Text Solution
|
- Out of different state parameters like E, H, G, A and S, only entropy(...
Text Solution
|
- Out of different state parameters like E, H, G, A and S, only entropy(...
Text Solution
|
- When acids an bases react they liberate some amount of energy which is...
Text Solution
|
- When acids an bases react they liberate some amount of energy which is...
Text Solution
|
- Fuel cells are the commercial cells which converts the chemical energy...
Text Solution
|
- Fuel cells are the commercial cells which converts the chemical energy...
Text Solution
|
- Fuel cells are the commercial cells which converts the chemical energy...
Text Solution
|
- While analyzing driving forces for a chemical reaction it is observed ...
Text Solution
|
- While analyzing driving forces for a chemical reaction it is observed ...
Text Solution
|
- It is observed that when acid and bases react, some energy is released...
Text Solution
|
- It is observed that when acid and bases react, some energy is released...
Text Solution
|
- It is observed that when acid and bases react, some energy is released...
Text Solution
|
- For a process top be spontaneous, at constant temperature and pressure...
Text Solution
|
- For a process top be spontaneous, at constant temperature and pressure...
Text Solution
|
- For a process top be spontaneous, at constant temperature and pressure...
Text Solution
|
- For a process top be spontaneous, at constant temperature and pressure...
Text Solution
|
- For a process top be spontaneous, at constant temperature and pressure...
Text Solution
|
- Standard Gibb's energy of reaction (Delta(r )G^(@)) at a certain temp...
Text Solution
|
- Enthalpy of neutralzation is defined as the enthalpy change when 1 mol...
Text Solution
|