Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-THERMODYNAMICS-All Questions
- In a particular case of physisorption, magnitude of enthalpy change an...
Text Solution
|
- Melting point of any solid depends on pressure as (P(2)-P(1))=(DeltaH(...
Text Solution
|
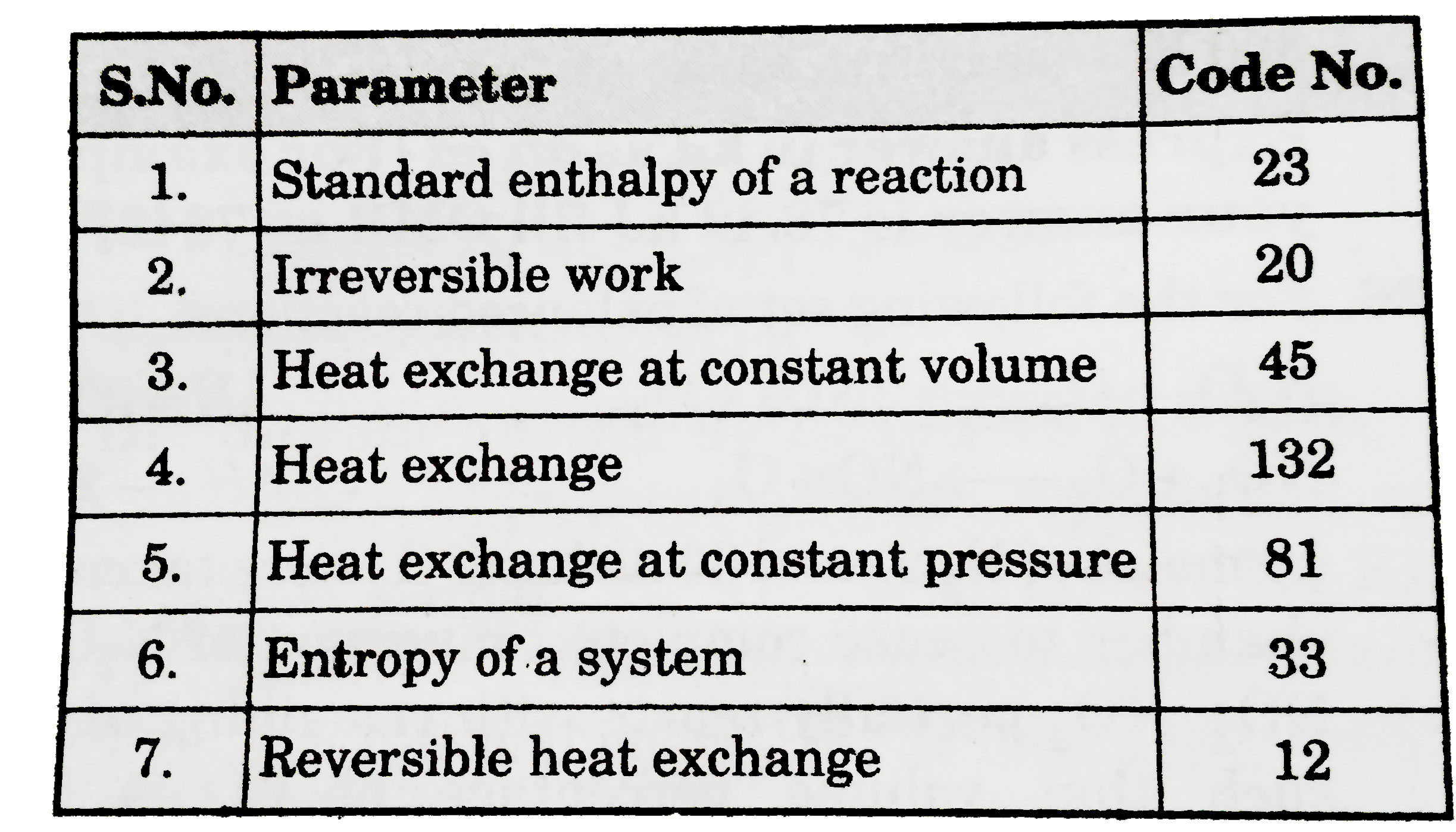
- Calculate the sum of "code numbers" of all those parameters which are ...
Text Solution
|
- A defintie amount of diatomic ideal gas undergoes reversible adiabatic...
Text Solution
|
- A sample of an ideal gas is expanded 1 m^(3) to 3 m^(3) in a reversibl...
Text Solution
|
- Find out the heat evolved in combustion if 112 litre (at 1 atm, 273 K)...
Text Solution
|
- Calculate DeltaH^(@) ("in" kJmol^(-1)) for the reaction CH(2)Cl(2)(...
Text Solution
|
- Calculate the enthalpy change (DeltaH) in KJ mol^(-1), of the follwing...
Text Solution
|
- Calculate free energy change for the reaction: H(2)(g) + CI(2)(g) ra...
Text Solution
|
- The increase in entropy of 1 kg of ice at 200 K which is heated to 400...
Text Solution
|
- Calculate the amount of heat evolved during the complete combustion o...
Text Solution
|
- For the real gases reaction, 2CO(g)+O(2)(g)to2CO(2)(g),DeltaH=-560 k...
Text Solution
|
- In a constant volume calorimeter, 3.5 g of a gas with molecular weight...
Text Solution
|
- Fixed amount of an ideal monoatomic gas contained in a seeled rigid ve...
Text Solution
|
- At298 K,DeltaH("combustion")^(@)("sucrose")=-5737"kJ"//"mol" and Delta...
Text Solution
|
- What is the total number of intensive properties in the giben list ? ...
Text Solution
|
- For the reaction 2A(g)+3B(l)rarrC(g)+4D(l),DeltaH=300"cal//mol" Ca...
Text Solution
|
- One mole of an ideal monoatomic gas at 27^(@)C undergoes the process i...
Text Solution
|
- A certain gas in expanded from (1L, 10 atm) to (4L, 5 atm) against a c...
Text Solution
|
- How many times volume of diatomic gas should be expanded reversibly an...
Text Solution
|