Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-THERMODYNAMICS-All Questions
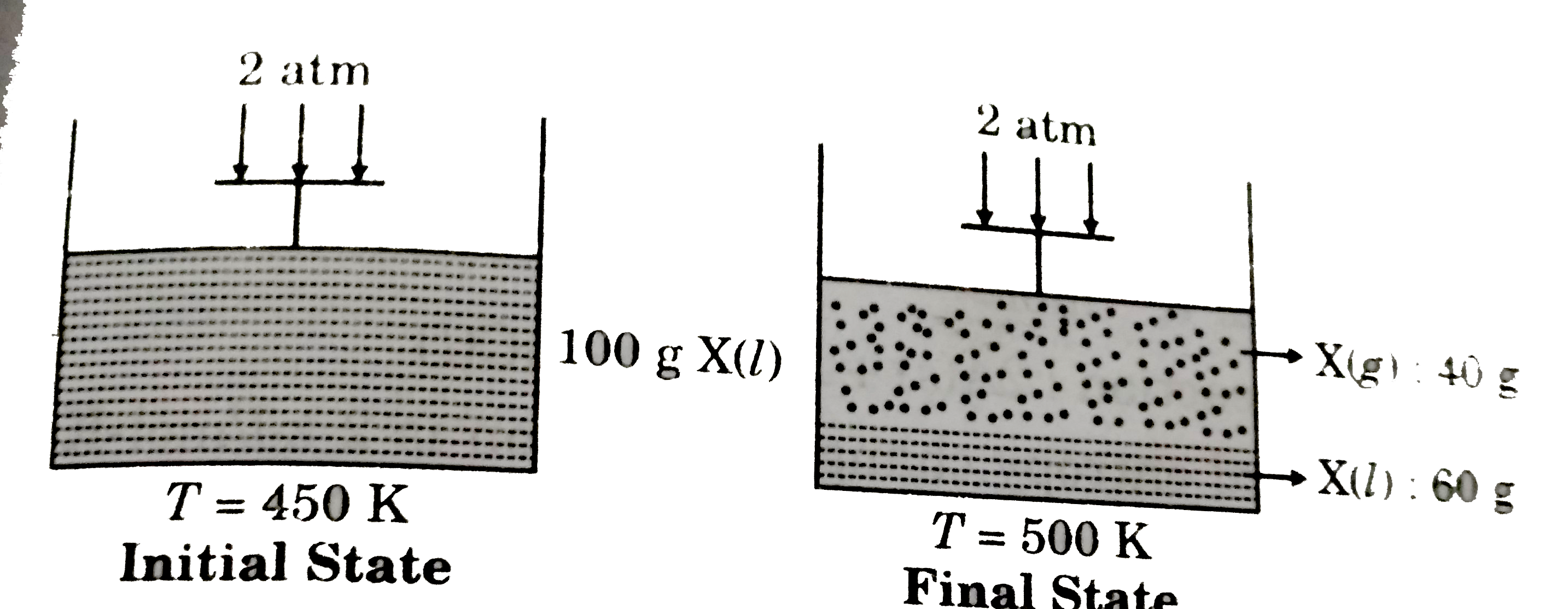
- The external pressure 2 atm is applied on frictionless movable piston,...
Text Solution
|
- 1 mole of argon is expanded isothermiocally and irreversably ( not aga...
Text Solution
|
- Select the correct set of "statement"//s : (P) Work done by the surr...
Text Solution
|
- A typical adults needs 33 kcal per kg body weight per day. Assuming an...
Text Solution
|
- In an isothermical expansion of a gaseous sample the correct relation ...
Text Solution
|
- a monoatomic gas (Cv=(3)/(2)R) is allowed to expand adiabaticaly and r...
Text Solution
|
- An ideal gas with C(v)=3 R expands adiabatically into a vaccum thus do...
Text Solution
|
- What is the work done against the atmosphere when 25 grams of water va...
Text Solution
|
- A certain mass of gas expanded from ( 1L, 10 atm) to (4 L, 5 atm) agai...
Text Solution
|
- A container of volume 2L is seperated into equal compartments. In one ...
Text Solution
|
- Which of the following options is not correct w.r.t. van der Waal's ga...
Text Solution
|
- 5 moles of an ideal monoatomic gas absorbes x joule when heated from 2...
Text Solution
|
- The only incorrect statement for the value of gamma for NH(3) gas is :...
Text Solution
|
- For a gaseous reaction, 2SO(2)+O(2)to2SO(3),DeltaH=-440kJ//"mole" ...
Text Solution
|
- For which of the following process abs(DeltaH)ltabs(DeltaE) :
Text Solution
|
- For a fixed amouunt of an ideal gas(gamma=(11)/(9)), the change in int...
Text Solution
|
- Calculate change in enthalpy when 2 moles of liquid water at 1 bar and...
Text Solution
|
- Which of the following statement is incorrect regarding adiabatic and ...
Text Solution
|
- Calculate work involved in compression of 2 moles of H(2) gas reversib...
Text Solution
|
- Caalculate DeltaHwhen 2 moles of solid benzoic acid undergo complete c...
Text Solution
|
- For the combustion of CH(4) at 1 atm pressure and 300 K, which of the ...
Text Solution
|