Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-THERMODYNAMICS-All Questions
- For a reaction 2A(g)+3B(g) to 2C(g)+D(g), calculate the magnitude of D...
Text Solution
|
- 12.5 millinmole of NH(4)NO(3) dissolved in enough water to make 25.0 m...
Text Solution
|
- A gas expands from a valume of 3 dm^(3) " to " 30 dm^(3) against a con...
Text Solution
|
- Calculate DeltaG (in bar-L) when a definite mass of a monoatomic ideal...
Text Solution
|
- How much heat (in kJ) should be supplied to a rigid conducting vessel ...
Text Solution
|
- A gaseous reaction A(g) hArrB(g) is at equilibrium under standard con...
Text Solution
|
- At 500 kbar pressure density of diamond and graphite are 3 "g"//"cc" ...
Text Solution
|
- Calculate DeltaG(reaction) ("kJ"//"mol") for the given reaction at 300...
Text Solution
|
- An imaginary engine, is capable of expanding the gas upto 10^(13) time...
Text Solution
|
- The heat evolved on combustion of 1 gm of starch, (C(6)H(10)O(5))x, in...
Text Solution
|
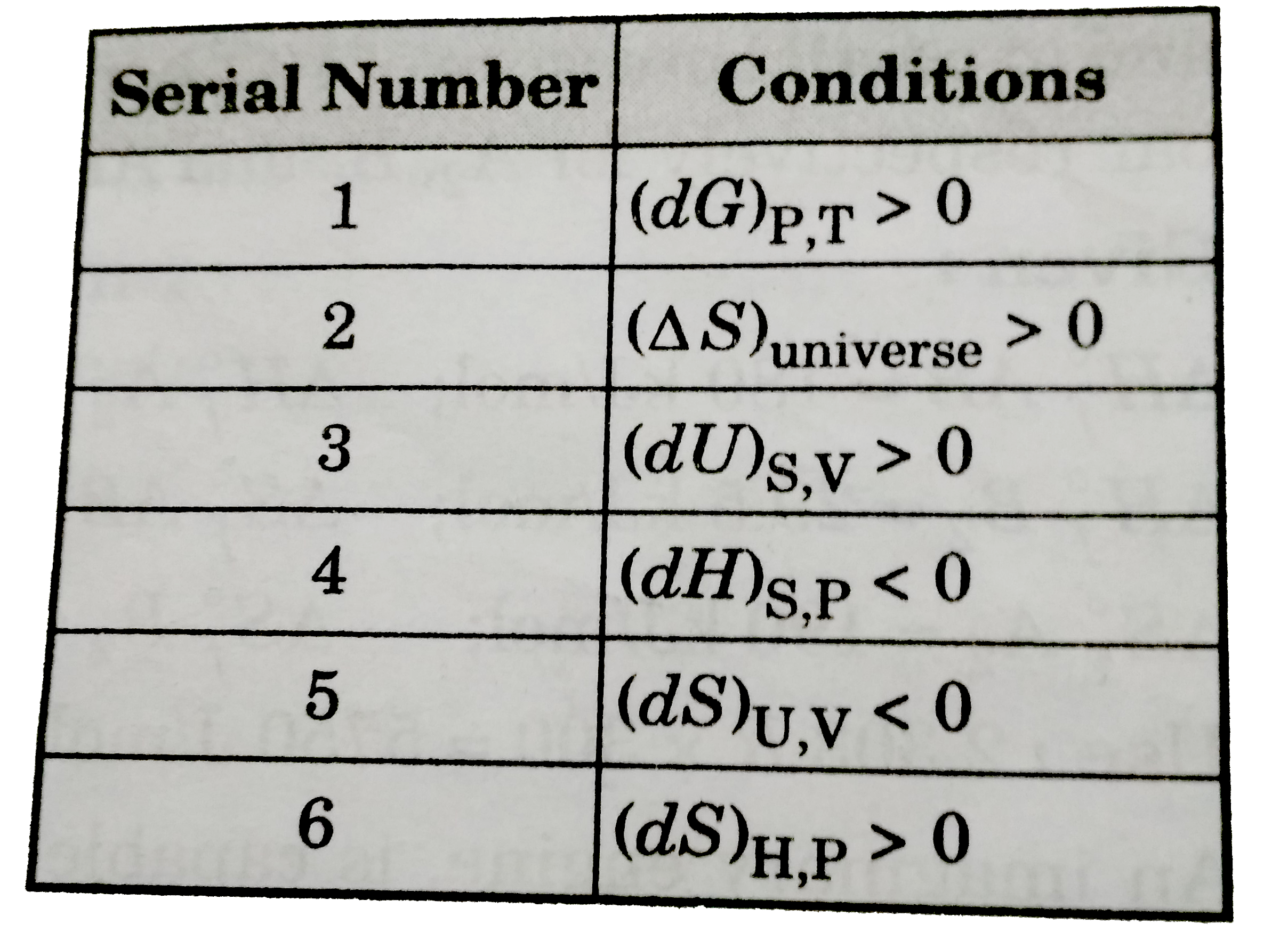
- Consider the following six conditions. (Serial number 1 to6). Select t...
Text Solution
|
- Calculate Delta G (in joule) for the reaction 2A(g) to B(g)+C(g) w...
Text Solution
|
- 4 mole H(2)O(g),2.5mole H(2), 2.5 mole CO(g) and 1 mole inert gas He a...
Text Solution
|
- For the reaction, N(2)(g)+O(2)(g)hArr2NO(g), " " Delta G^(@)=18.6 ...
Text Solution
|
- Calculate the magnitude of ring strain energy in ("kJ"//"mol") of cycl...
Text Solution
|
- The standard molar enthalpies of formation of H(2)O(l) " and " H(2)O(2...
Text Solution
|
- The standard molar enthalpies of formation of IF(3)(g)" and " IF(5)(g...
Text Solution
|
- Ethalpy for the reaction Ag^(+)(aq)+Br^(-)(aq)toAgBr(s) " is" -90 kJ...
Text Solution
|
- For the reaction 2H(2)(g)+ O(2)(g)to 2H(2)O(l). The standard entropie...
Text Solution
|
- Enthalpy of neutralization of H(3)PO(3) "with " NaOH " is" -106.68 "kJ...
Text Solution
|