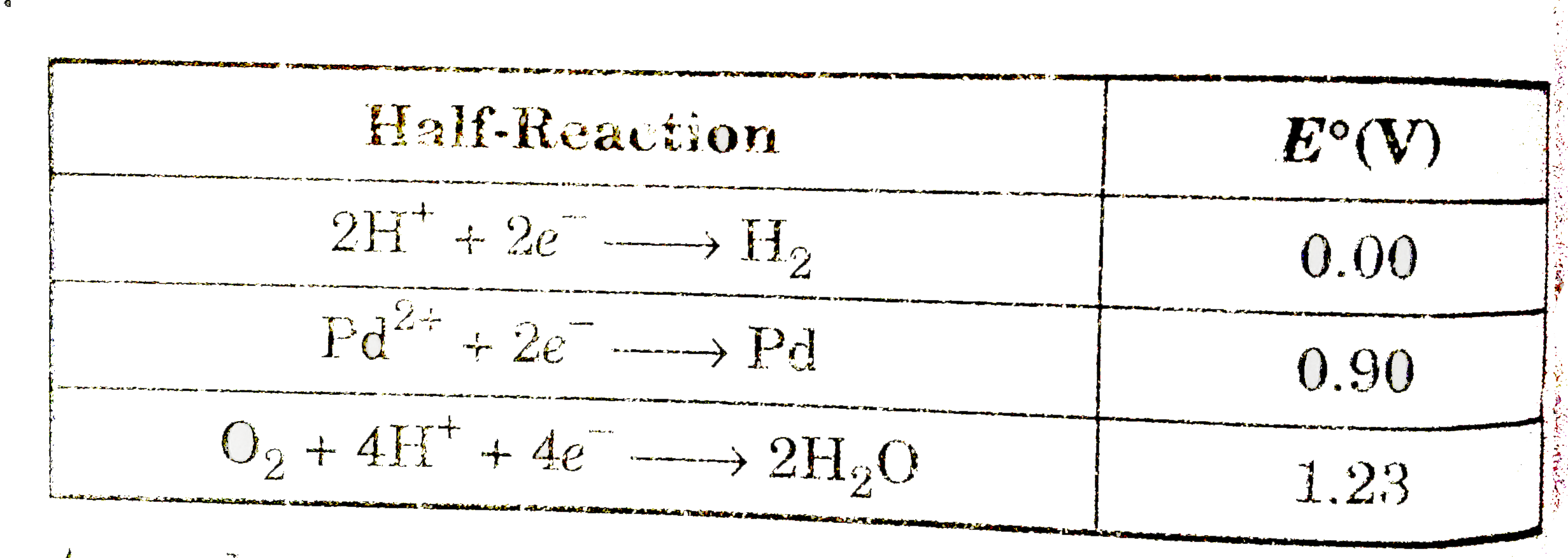
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTY
GRB PUBLICATION|Exercise Latimer Diagram, concentration cells|117 VideosELECTROCHEMISTY
GRB PUBLICATION|Exercise Batteries|10 VideosELECTROCHEMISTY
GRB PUBLICATION|Exercise Subjective type|39 VideosD-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Subjective Type|18 VideosENVIRONMENTAL CHEMISTRY
GRB PUBLICATION|Exercise Straight objective type|40 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-ELECTROCHEMISTY-Galvanic Cell and Salt Bridge
- Consider the following reactions: X(NO(3)(2) + Y rightarrow X + Y(NO...
Text Solution
|
- When connected to a Standard Hydrogen Electrode (SHE) electrons flow f...
Text Solution
|
- Given these standards reduction potentials, what is the free energy ch...
Text Solution
|
- Given the standard reduction potentials, which statement is correct? ...
Text Solution
|
- In a galvanic cell in which the following spontaneous reaction takes p...
Text Solution
|
- For a galvanic cell involving the half-reaction at standard conditions...
Text Solution
|
- According to the half-reaction table, Sn^(2+) + 2e^(-) rightarrow Sn...
Text Solution
|
- The reduction potentials for the +2 cations, e.g. A^(2+) + 2e^(-) ri...
Text Solution
|
- E^(@) = 0.93V for the reactions: Fe(s) + 2M^(+)(aq) rightarrow Fe^(2...
Text Solution
|
- In the galvanic cell shown below, which arrow indicates the spontaneou...
Text Solution
|
- What is the standard cell potential for the voltaic cell? Cr || Cr^(...
Text Solution
|
- What is the E^(@) value for the voltaic cell constructed from the half...
Text Solution
|
- Based on the reactions and data below, which reaction is least spontan...
Text Solution
|
- Use the standard reduction potentials: Sn^(2+)(aq) + 2e^(-) rightarr...
Text Solution
|
- What is the standard cell potential for the reaction, 2Cr(s) + 3Sn^(...
Text Solution
|
- Which is the weakest oxidizing agent in a 1 M aqueous solution?
Text Solution
|
- Consider the cell potentials E(Mg^(2+)|Mg)^(0)=-2.37V and E(Fe^(3+)|Fe...
Text Solution
|
- Use the E^(@) values in the table above to the determining which of th...
Text Solution
|
- According to the equations and data in the table above, which species ...
Text Solution
|
- According to the standard reduction potentials above, cathodic protect...
Text Solution
|