A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
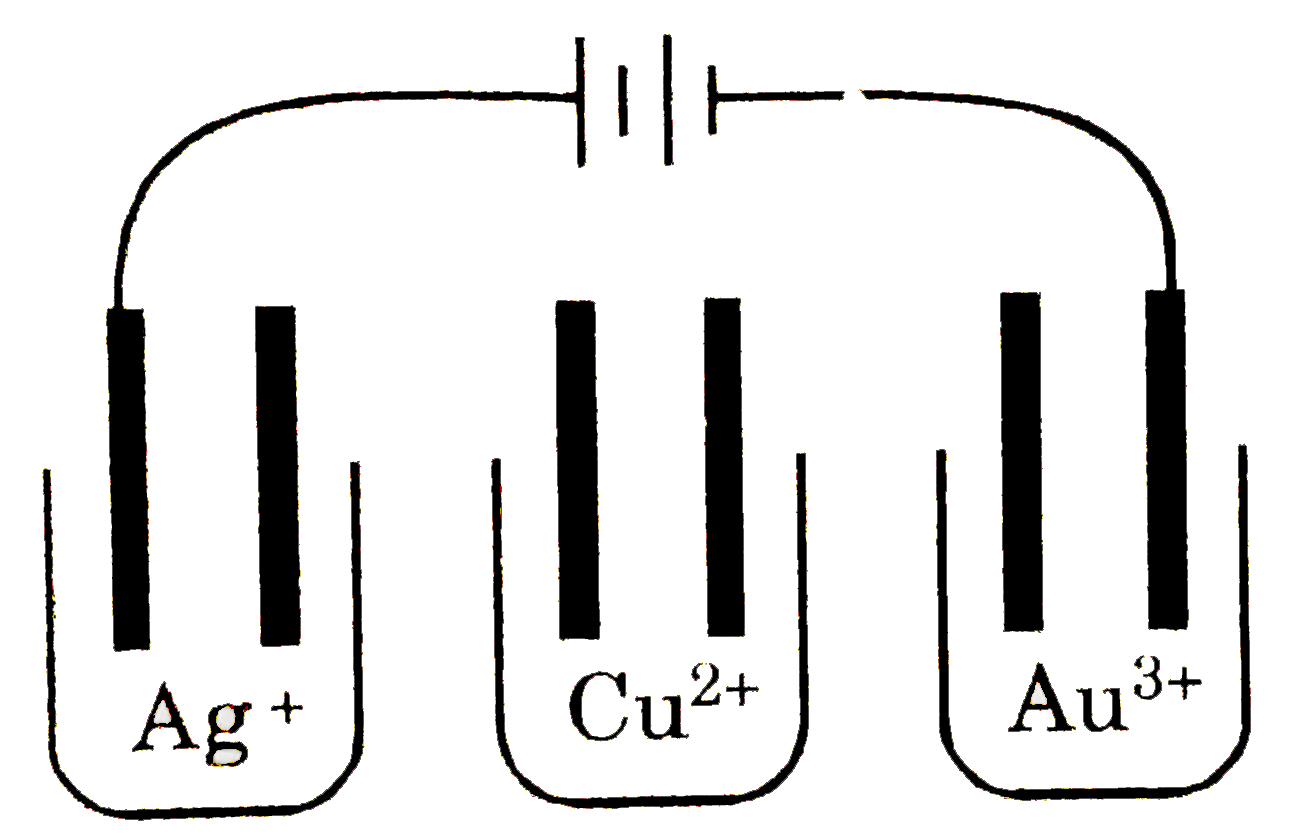
- Solutions of AgNO(3),CuSO(4) and AuCl(3) are electrolyzed in the appar...
Text Solution
|
- A solution of CuSO(4) is electrolyzed for 10 min with a current of 1....
Text Solution
|
- 1.0 M aqueous solutions of AgNO(3), Cu(NO(3))(2) and Au(NO(3))(3) are ...
Text Solution
|
- Solutions of AgNO(3),CuSO(4) and AuCl(3) are electrolyzed in the appar...
Text Solution
|
- If three faradyas of electricity is passed through the solutions of Ag...
Text Solution
|
- The same quantity of electrical charge deposited 0.583g of Ag when pas...
Text Solution
|
- Two electrolytic cells are connected in series containing CuSO(4) solu...
Text Solution
|
- If same amount of electricity is passed through aqueous solutions of A...
Text Solution
|
- In an aqueous solution AgNO(3) and CuSO(4) are connected in seri...
Text Solution
|