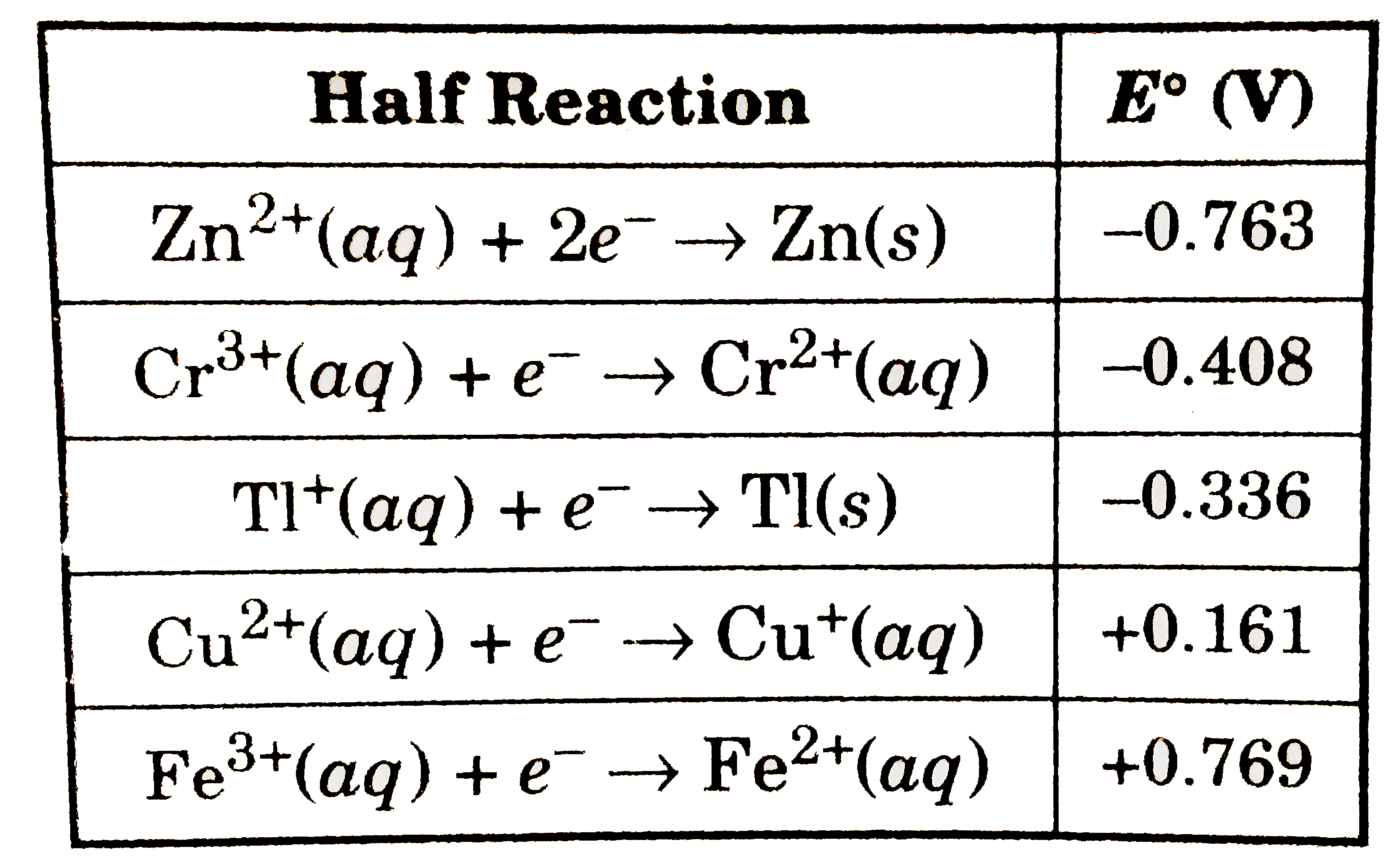
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTY
GRB PUBLICATION|Exercise Match the column type|16 VideosELECTROCHEMISTY
GRB PUBLICATION|Exercise Subjective type|39 VideosELECTROCHEMISTY
GRB PUBLICATION|Exercise Multiple objective type|33 VideosD-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Subjective Type|18 VideosENVIRONMENTAL CHEMISTRY
GRB PUBLICATION|Exercise Straight objective type|40 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-ELECTROCHEMISTY-Comprehension type
- Ag^(+)(aq)+e^(-)rarrAg(s) E^(@)=0.80V Cu^(2+)(aq)+2e^(-)rarrCu(s) E^...
Text Solution
|
- Rh^(03+)(aq)+3e^(-)rarrRh(s) E^(@)=0.80V Cu^(+)(aq)+e^(-)rarrCu(s) E...
Text Solution
|
- Rh^(03+)(aq)+3e^(-)rarrRh(s) E^(@)=0.80V Cu^(+)(aq)+e^(-)rarrCu(s) E...
Text Solution
|
- Al^(3+)(aq)+3e^(-)rarrAl(s) E^(@)=-1.66V Mn^(2+)(aq)+2e^(-)rarrMn(s)...
Text Solution
|
- Al^(3+)(aq)+3e^(-)rarrAl(s) E^(@)=-1.66V Mn^(2+)(aq)+2e^(-)rarrMn(s)...
Text Solution
|
- 2Al(s)+2Cu^(2+)(aq)rarr2Al^(3+)(aq)+2Cu(s) What is the value of E^(@...
Text Solution
|
- 2Al(s)+2Cu^(2+)(aq)rarr2Al^(3+)(aq)+2Cu(s) What value should be used...
Text Solution
|
- Use the standard reduction potentials to find the standard cell potent...
Text Solution
|
- Based on the standard reduction potentials above, which reaction(s) is...
Text Solution
|
- 2Ag^(+)(aq)+M(s)rarrM^(2+)(aq)+2Ag,E^(@)=0.940V What is the value of...
Text Solution
|
- 2Ag^(+)(aq)+M(s)rarrM^(2+)(aq)+2Ag,E^(@)=0.940V Which change will ca...
Text Solution
|
- 0.1 "mole" AgNO(3) is added in 250 mL of saturated solution of AgCl at...
Text Solution
|
- 0.1 "mole" AgNO(3) is added in 250 mL of saturated solution of AgCl at...
Text Solution
|
- 0.1 "mole" AgNO(3) is added in 250 mL of saturated solution of AgCl at...
Text Solution
|
- H(2)O(2) can be produced by the ammonium hydrogen sulphate. Reactions ...
Text Solution
|
- H(2)O(2) can be produced by the ammonium hydrogen sulphate. Reactions ...
Text Solution
|
- H(2)O(2) can be produced by the ammonium hydrogen sulphate. Reactions ...
Text Solution
|
- An element (X) having same cheical properties as that of hydrogen atom...
Text Solution
|
- An element (X) having same cheical properties as that of hydrogen atom...
Text Solution
|
- An element (X) having same cheical properties as that of hydrogen atom...
Text Solution
|