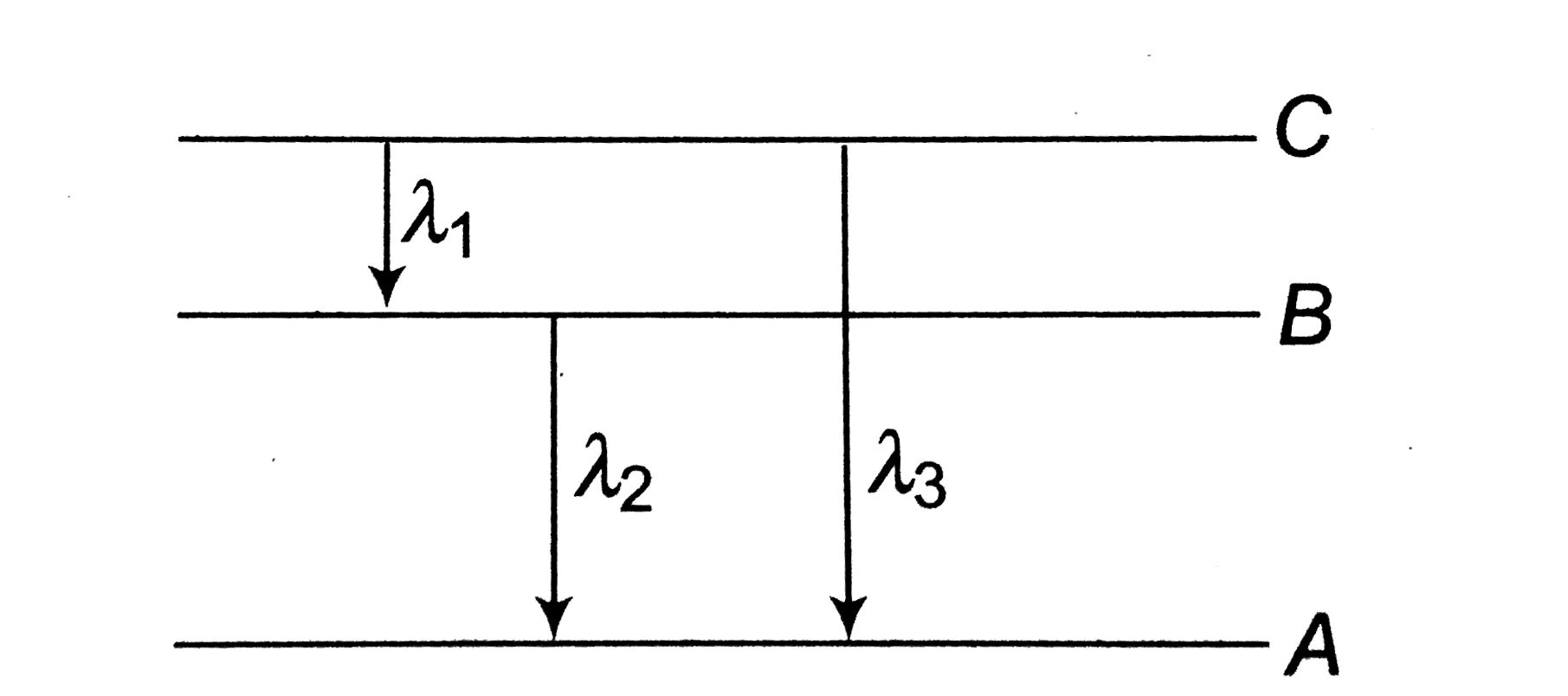
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS-ATOMIC PHYSICS-dpp 4.2
- The energy levels of the hydrogen spectrum is shown in figure. There a...
Text Solution
|
- In the figure of previous problem, D and E respectively represent
Text Solution
|
- If the wavelength of the first line of the Balmer series of hydrogen i...
Text Solution
|
- The enegry level diagram for an hydrogen-like atom is shown in the fig...
Text Solution
|
- If the series limit of Lyman series for Hydrogen atom is equal to the ...
Text Solution
|
- The following diagram indicates the energy levels of a certain atom wh...
Text Solution
|
- A hydrogen atom emits a photon corresponding to an electron transition...
Text Solution
|
- Energy levels A, B, C of a certain atom corresponding to increasing va...
Text Solution
|
- Figure shows the enegry levels P, Q, R, S and G of an atom where G is ...
Text Solution
|
- The figure indicates the enegry level diagram of an atom and the origi...
Text Solution
|
- A hydrogen like atom with atomic number Z is in an excited state of q...
Text Solution
|
- The energy, the magnitude of linear momentum, magnitude of angular mom...
Text Solution
|
- A single electron orbits a stationary nucleus of charge + Ze, where Z ...
Text Solution
|
- A single electron orbit around a stationary nucleus of charge + Ze whe...
Text Solution
|
- A single electron orbit around a stationary nucleus of charge + Ze whe...
Text Solution
|