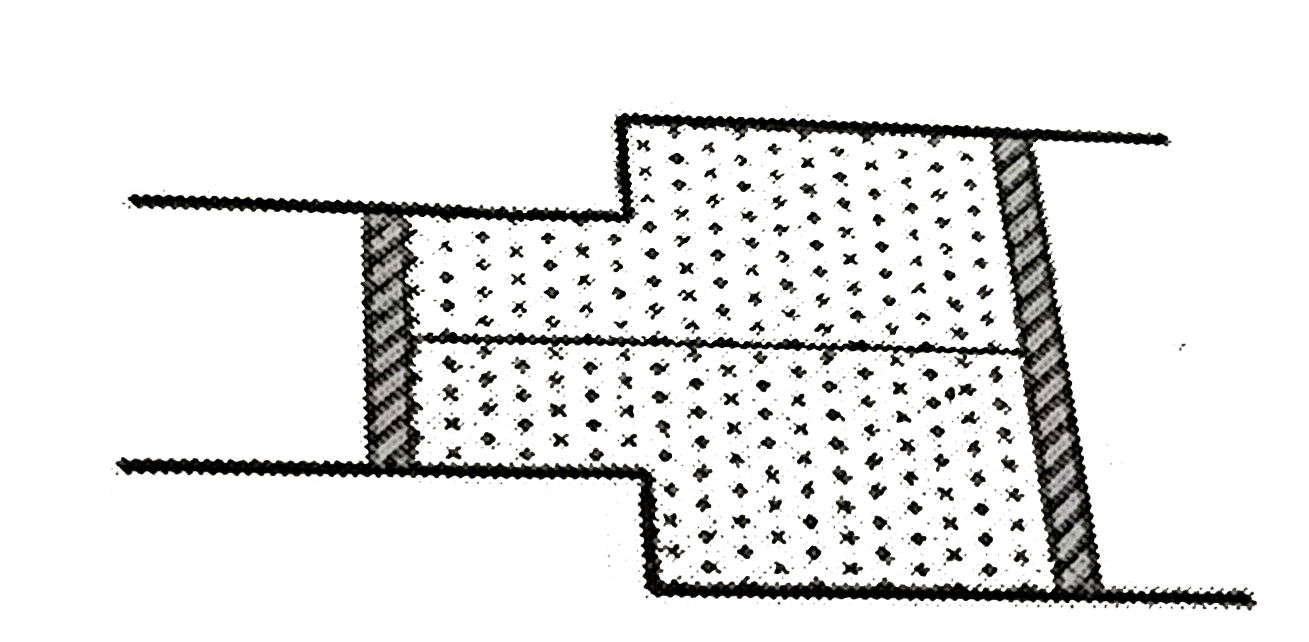
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
D MUKHERJEE-HEAT AND THERMODYNAMICS-All Questions
- Two idential container joined by a small pipe initially contain the sa...
Text Solution
|
- In the previous question let V(0) be the volume of each container. All...
Text Solution
|
- A horizontal cylinder has two sections of unequal cross - sections, in...
Text Solution
|
- A gas expands from 1 litre to 3 litres at atmospheric pressure. The wo...
Text Solution
|
- During an adiabatic process, the pressure of a gas is found to be prop...
Text Solution
|
- In the previous question, the gas may be
Text Solution
|
- Each molecule of a gas has F degrees of freedom . The ratio (C(p))/(C(...
Text Solution
|
- A mixture of n(1) moles of monoatomic gas and n(2) moles of diatomic g...
Text Solution
|
- The pressure p for a gas is plotted against its absolute temperature T...
Text Solution
|
- A cyclic process ABCD is shown in the p-V diagram. Which of the follow...
Text Solution
|
- The ratio (Cp)/(Cv)=gamma for a gas. Its molecular weight is M. Its sp...
Text Solution
|
- When an ideal gas undergoes an adiabatic change causing a temperature ...
Text Solution
|
- When an ideal diatomic gas is heated at constant pressure, the fractio...
Text Solution
|
- The average degrees of freedom per molecule for a gas are 6. The gas p...
Text Solution
|
- Two cylinders A and B fitted with pistons contain equal amounts of an ...
Text Solution
|
- A system is taken from state A to state B along two different paths 1 ...
Text Solution
|
- Equal masses of three liquids A, B and C have temperature 10^(@)C, 25^...
Text Solution
|
- If water at 0^@C kept in a container with an open top, is placed a lar...
Text Solution
|
- In the previous question, if the specific latent heat of vaporization ...
Text Solution
|
- A substance of mass M kg requires a power input of P wants to remain i...
Text Solution
|