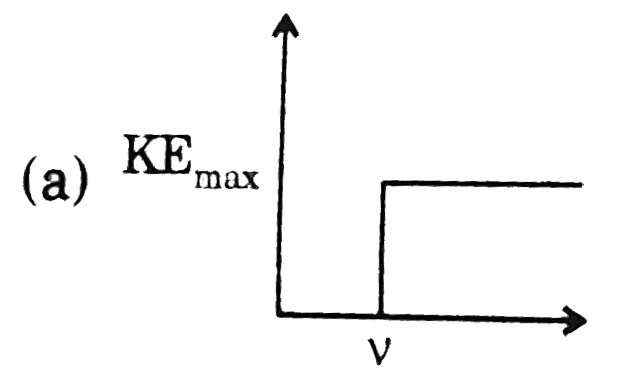
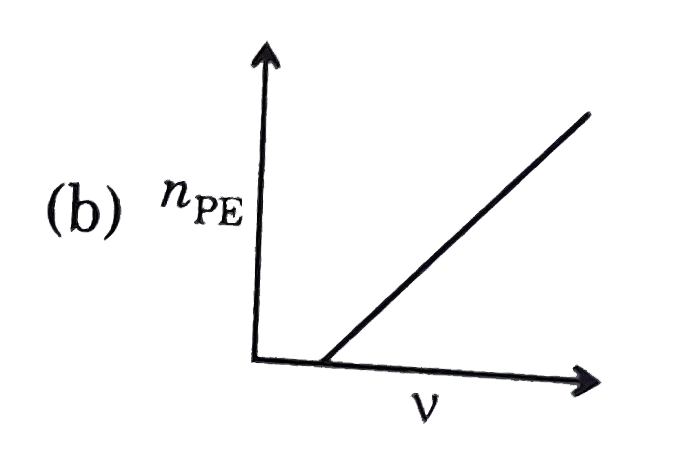
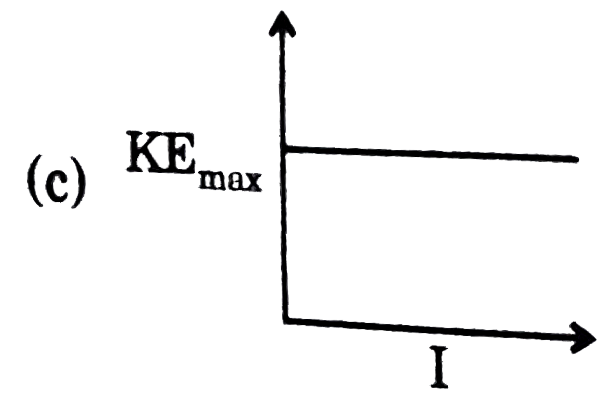
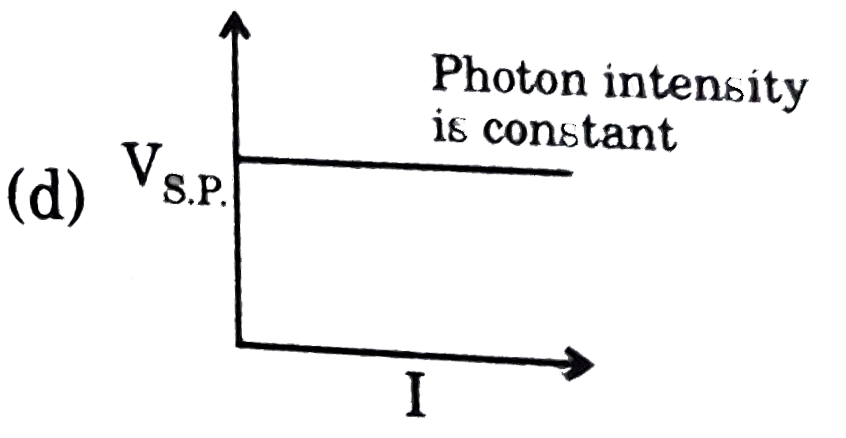
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise D. Liquid Solutions|1 VideosGRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise Liquid Solutions|12 VideosGRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise C. Atomic Sturcture|1 VideosF-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Subjective type|7 VideosHYDROCARBON (ALIPHATIC)
GRB PUBLICATION|Exercise Previous years jee questions|28 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-GRAPHICAL INTERPRETATION-Atomic Sturcture
- Consider the following radial distribution function diagrams. Which of...
Text Solution
|
- A graph is plotted between uncertainty in position and inverse of unce...
Text Solution
|
- For a H-like species if area A(1) is of ground state orbit and area A(...
Text Solution
|
- Which of the following plots of radical probability function 4pi r^(2)...
Text Solution
|
- Which of the following radial probability distribution graph is correc...
Text Solution
|
- Which of the following graphs is correct with respect to phtotelectric...
Text Solution
|
- The radial probability distribution curve for an orbitals comprises of...
Text Solution
|
- Given curve represents
Text Solution
|
- Which of the following will be correct graph for variation of boiling ...
Text Solution
|