A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise H. Chemical Kinetics|1 VideosGRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise Chemical Kinetics|80 VideosGRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise G. Chemical Equilibrium|1 VideosF-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Subjective type|7 VideosHYDROCARBON (ALIPHATIC)
GRB PUBLICATION|Exercise Previous years jee questions|28 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-GRAPHICAL INTERPRETATION-Chemical Equilibrium
- In the dissociation of N(2)O(4) into NO(2)O(4) into NO(2), alpha varie...
Text Solution
|
- For the chemical equilibrium, CaCO(3)(s) hArr CaO(s)+CO(2)(g) Delt...
Text Solution
|
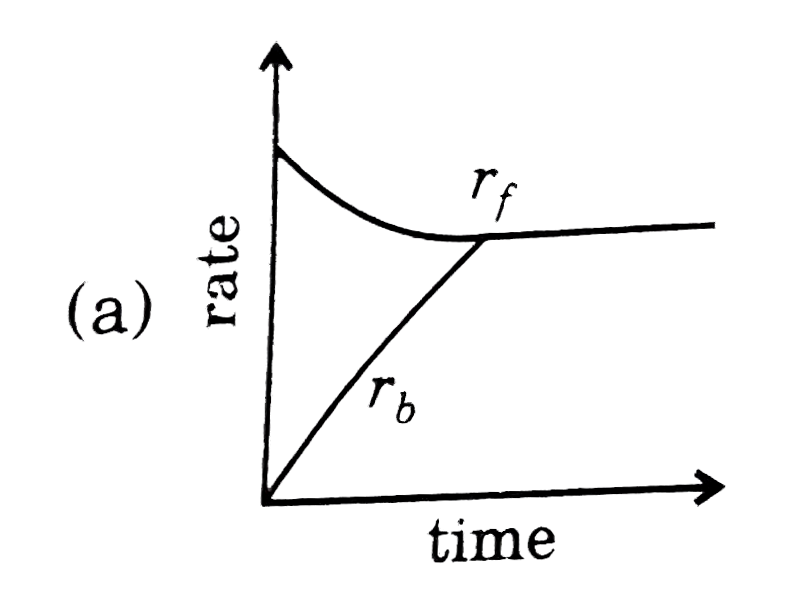
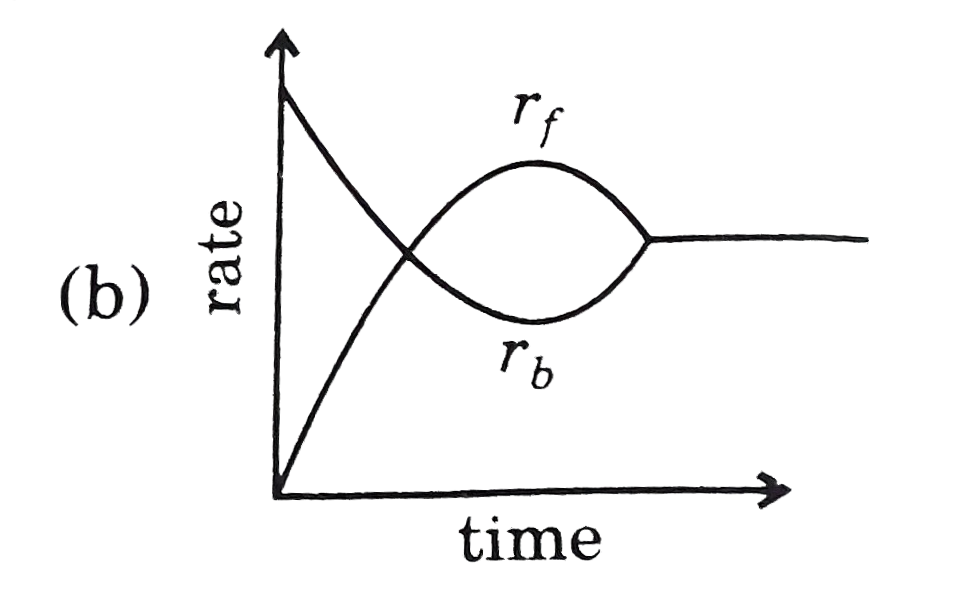
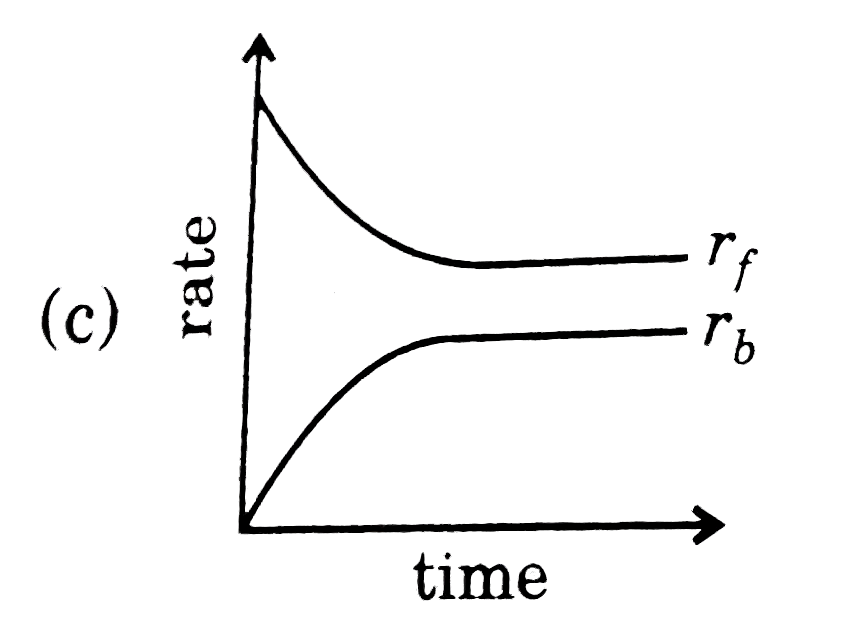
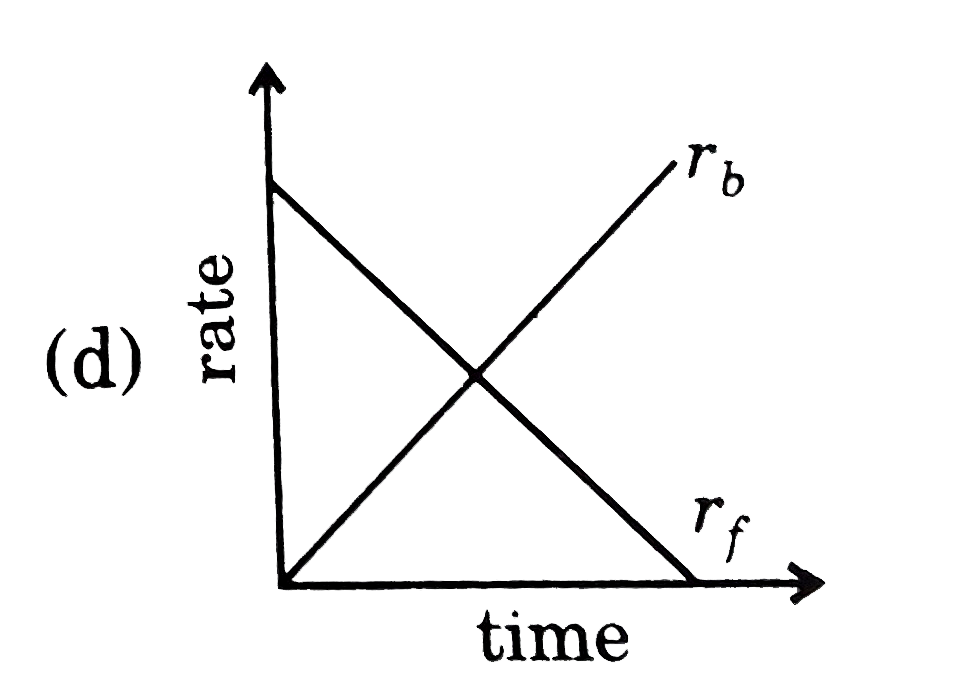
- Rate of reaction curve for equilibrium can be like: [r(f)= forward rat...
Text Solution
|
- N(2)O(4)(g) hArr 2NO(2)(g), K(c) = 4. This reversible reaction is stud...
Text Solution
|
- For the equilibrium, CH(3)-CH(2)-CH(2)-CH(3)(g) hArr CH(3)-overset(...
Text Solution
|
- For the given endothermic reaction, A(g) hArr 2B(g) The variation in c...
Text Solution
|
- A particular reversible reaction shifts towards reactant side if tempe...
Text Solution
|
- Which plot involving vapour pressure (VP) and absolute temperature res...
Text Solution
|
- According to the phase diagram of methanol shown below, which statemen...
Text Solution
|
- The phase diagram for sulphur is shown below. Which statement about th...
Text Solution
|
- What quantity is represented by the slope of the line in this graph of...
Text Solution
|
- Water ice exists in several different form depending on the pressure a...
Text Solution
|
- Under certain conditions, CO(2) melts rather than sublimes, CO(2) whic...
Text Solution
|
- Which of the following graphs is the correct phase diagram of a substa...
Text Solution
|
- What is the normal melting point of the substance represented by the p...
Text Solution
|
- Which points in this phase diagram represent conditions of temperature...
Text Solution
|
- The curve shown results when a liquid is cooled. What temperature is c...
Text Solution
|
- According to the solubility curve shown, how many grams of solute can ...
Text Solution
|
- According to the phase diagram, what would be the effect of increasing...
Text Solution
|
- According to the phase diagram shown, in what state does not represent...
Text Solution
|