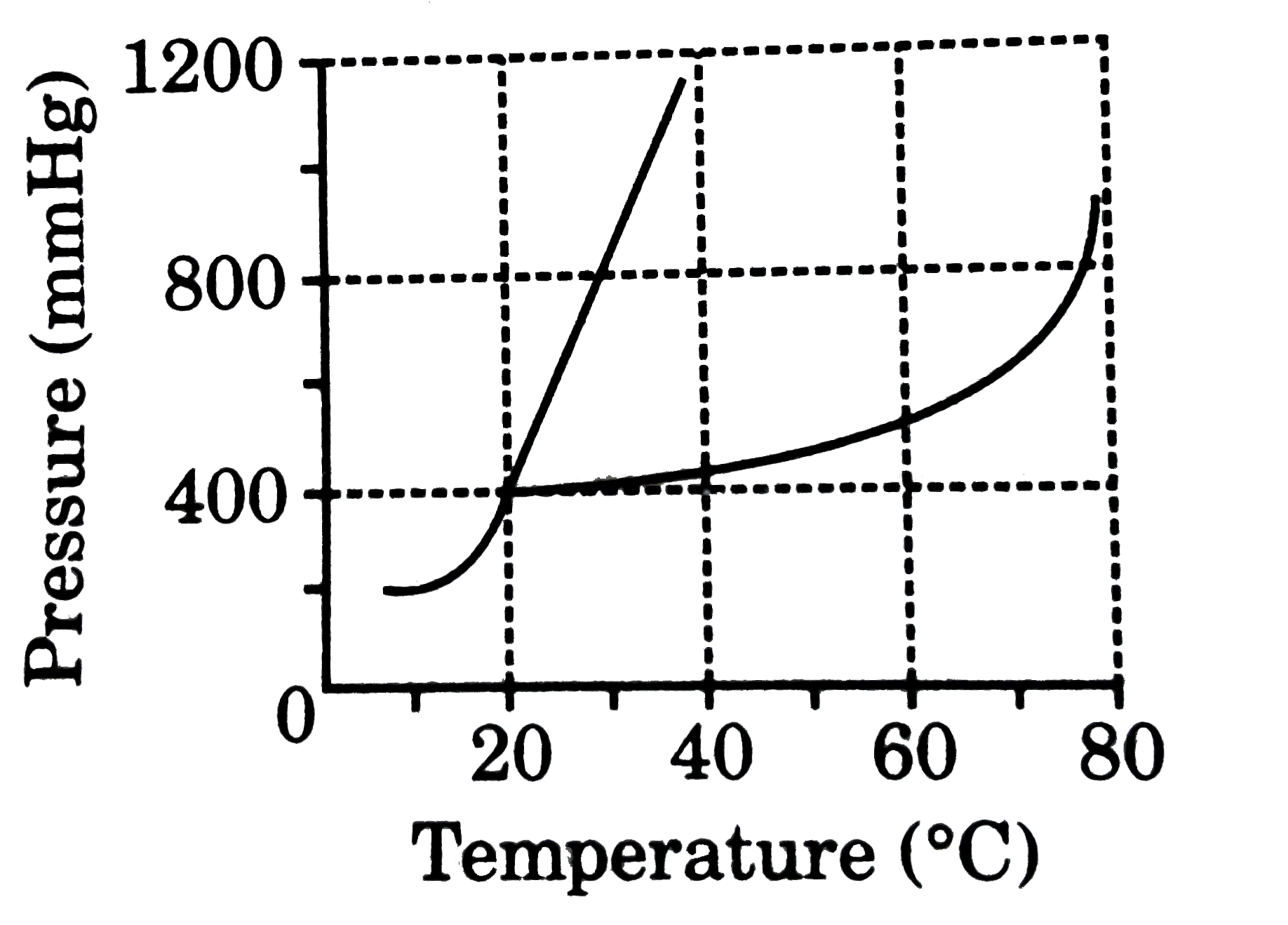
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise H. Chemical Kinetics|1 VideosGRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise Chemical Kinetics|80 VideosGRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise G. Chemical Equilibrium|1 VideosF-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Subjective type|7 VideosHYDROCARBON (ALIPHATIC)
GRB PUBLICATION|Exercise Previous years jee questions|28 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-GRAPHICAL INTERPRETATION-Chemical Equilibrium
- Water ice exists in several different form depending on the pressure a...
Text Solution
|
- Under certain conditions, CO(2) melts rather than sublimes, CO(2) whic...
Text Solution
|
- Which of the following graphs is the correct phase diagram of a substa...
Text Solution
|
- What is the normal melting point of the substance represented by the p...
Text Solution
|
- Which points in this phase diagram represent conditions of temperature...
Text Solution
|
- The curve shown results when a liquid is cooled. What temperature is c...
Text Solution
|
- According to the solubility curve shown, how many grams of solute can ...
Text Solution
|
- According to the phase diagram, what would be the effect of increasing...
Text Solution
|
- According to the phase diagram shown, in what state does not represent...
Text Solution
|
- Which statement is correct about the substance represented by this pha...
Text Solution
|
- According to this phase diagram, which phases can exist at pressure lo...
Text Solution
|
- According to the phase diagram shown, where does a mixture of solid an...
Text Solution
|
- Supercritical carbon dioxide exists at which point on the accompanying...
Text Solution
|
- Which point on the phase diagram represents the normal boiling point?
Text Solution
|
- What can be concluded about the substance represented by this phase di...
Text Solution
|
- Which segment of the heating curve obtained at constant pressure corre...
Text Solution
|
- Which graph best represents the vapour pressure of water as a function...
Text Solution
|
- According to the graph (ln vapour pressure us 1//T) what can be conclu...
Text Solution
|
- For a hypothetical reaction: A(g) +B(g) hArr C(g) +D(g) a graph betwee...
Text Solution
|
- Which graph will show equilibrium, R = P ?
Text Solution
|