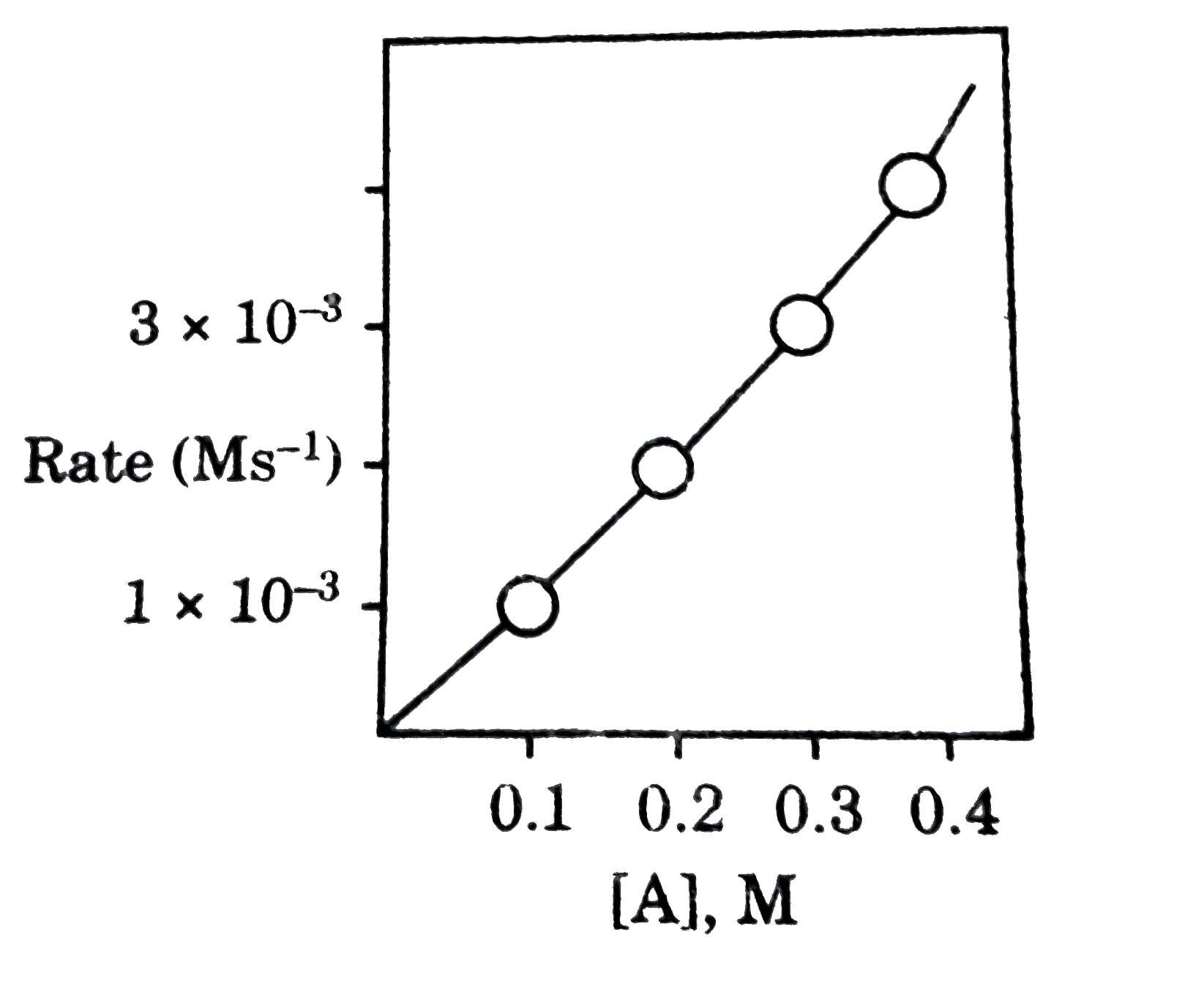
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise I.Electrochemistry|1 VideosGRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise Electrochemistry|9 VideosGRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise H. Chemical Kinetics|1 VideosF-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Subjective type|7 VideosHYDROCARBON (ALIPHATIC)
GRB PUBLICATION|Exercise Previous years jee questions|28 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-GRAPHICAL INTERPRETATION-Chemical Kinetics
- A simple mechanism for enzyme-catalysed reaction is given by the follo...
Text Solution
|
- In the following graphical representation for the reaction A rarr B th...
Text Solution
|
- Rate law of the reaction A rarr Product is, rate = k[A]. Graphically i...
Text Solution
|
- Which graph is diagnostic of an irreversible second order reaction A r...
Text Solution
|
- For the reaction A rarr B, what is the order with respect to A that gi...
Text Solution
|
- Which of the reactions represented in these diagrams will show the gre...
Text Solution
|
- What names apply to chemical species corresponding to locations 1 and ...
Text Solution
|
- How can be rate of reaction at a specific in the be determined from a ...
Text Solution
|
- If a reaction A rarr B has the rate low k[A],which graph produced a st...
Text Solution
|
- Following reaction can take place in both direction A overset(k(1))und...
Text Solution
|
- Graph between log k and (1)/(T) (k is rate constant in s^(-1) and T is...
Text Solution
|
- For a reaction A rarr B, E(a) = 10 kJ mol^(-1), DeltaH = 5 kJ mol^(-1)...
Text Solution
|
- Match the graphical study with the order of the reaction: A: Firs...
Text Solution
|
- The potential energy diagram for a reaction R rarr P is given DeltaH^(...
Text Solution
|
- For the first order reaction Following observation is made: wher...
Text Solution
|
- When a graph between log K and 1//T is drawn a straight line is obtain...
Text Solution
|
- The slope of straight line graph between ln k us (1)/(T) is equal to 2...
Text Solution
|
- For a reaction represented by A rarr B, which of the following options...
Text Solution
|
- For a certain reaction, a plot of ([C(0)-C])/(C ) against time t, yiel...
Text Solution
|
- Consider the following chart. Now with the following, select the cor...
Text Solution
|