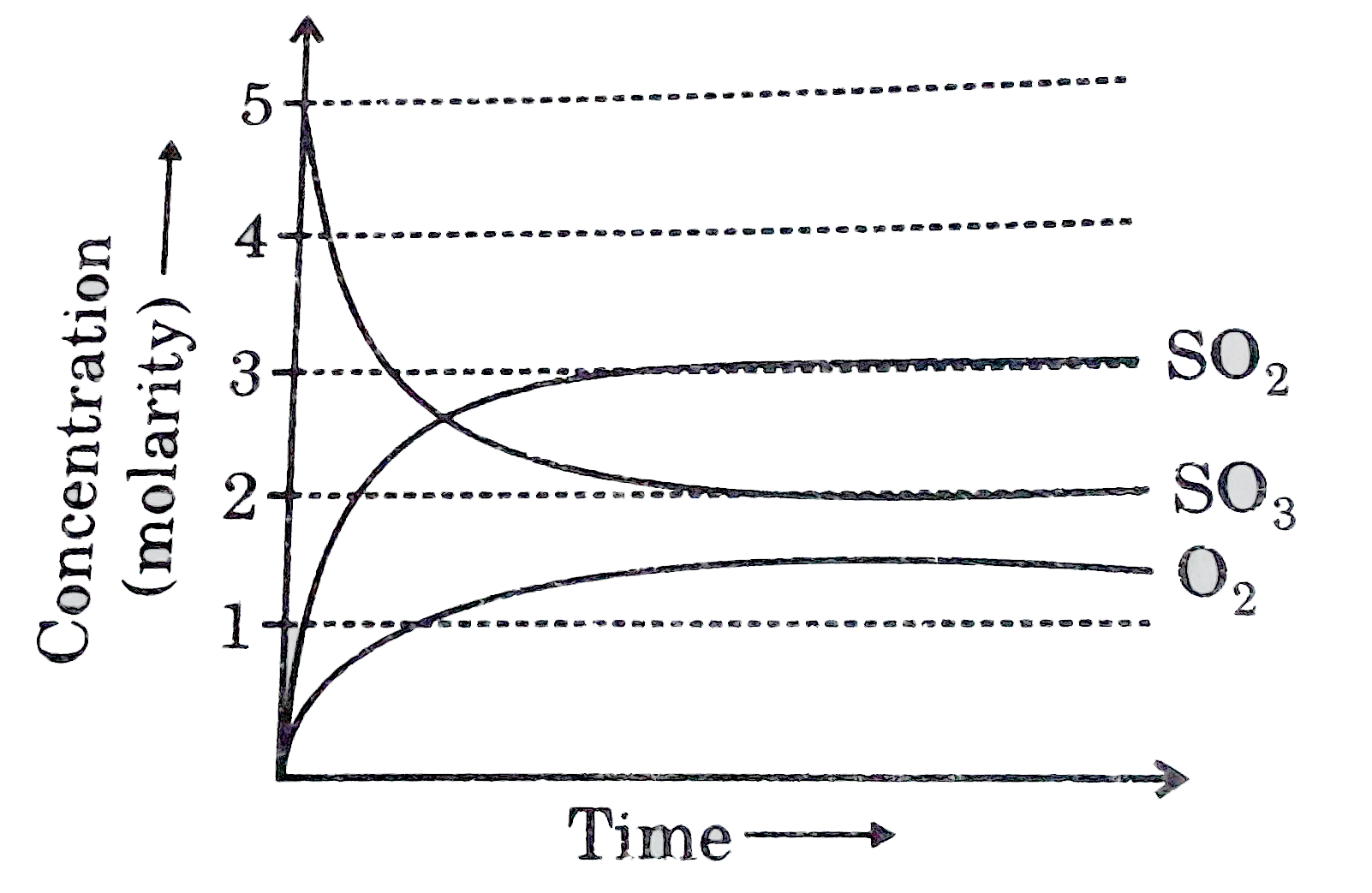
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise Comprehension 1|1 VideosGRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise Comprehension|49 VideosGRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise Electrochemistry|9 VideosF-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Subjective type|7 VideosHYDROCARBON (ALIPHATIC)
GRB PUBLICATION|Exercise Previous years jee questions|28 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-GRAPHICAL INTERPRETATION-Multiple Objective Type
- If critical temperature for the real gas is 500 K then the value of :
Text Solution
|
- Consider the following equilibrium A(g) hArr 2B(g) and the following g...
Text Solution
|
- The dissociation of SO(3)(g) into SO(2)(g) and O(2)(g) is carried out ...
Text Solution
|
- Which of the following statement (s) is/are correct with respect to Ps...
Text Solution
|
- The curve C is for the gas X at 273 K.Choose the correct statement (s)...
Text Solution
|
- For fixed amount of ideal gas In P (y-axis) us In B (x-axis) curve is ...
Text Solution
|
- For a van der Waals' gas, a = 4 atm-l^(2)//"mol"^(2) and b = 0.02l//"m...
Text Solution
|
- For the following process involving fixed moles of an ideal gas, selec...
Text Solution
|
- Which of the following graph is parabolic for fixed mass of an ideal g...
Text Solution
|
- For an ideal gas, following observation is made as per Maxwell-Boltzma...
Text Solution
|
- Which of the following statements is (are) true ?
Text Solution
|
- For a fixed mass of gas at constant volume, which of the following sta...
Text Solution
|
- The graph below shows the distribution of molecular speeds of two idea...
Text Solution
|
- Which of the following statement(s) are true about Z us P graph for a ...
Text Solution
|
- Which of following has correct matching of curve and orbital ?
Text Solution
|
- With refernce to above graph, which of the following is/are incorrect ...
Text Solution
|
- A gas has been subjected to an isochoric and isobaric cycle. Plot of t...
Text Solution
|
- An ideal gas is taken from the same initial pressure P(1) to the same ...
Text Solution
|
- In the graph showing Maxwell Boltzmann distribution of energy ...........
Text Solution
|
- In a chemical reaction A(g) is converted to B(g) following observation...
Text Solution
|