A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise Comprehension 2|1 VideosGRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise Comprehension 3|1 VideosGRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise Comprehension 1|1 VideosF-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Subjective type|7 VideosHYDROCARBON (ALIPHATIC)
GRB PUBLICATION|Exercise Previous years jee questions|28 Videos
GRB PUBLICATION-GRAPHICAL INTERPRETATION-Comprehension
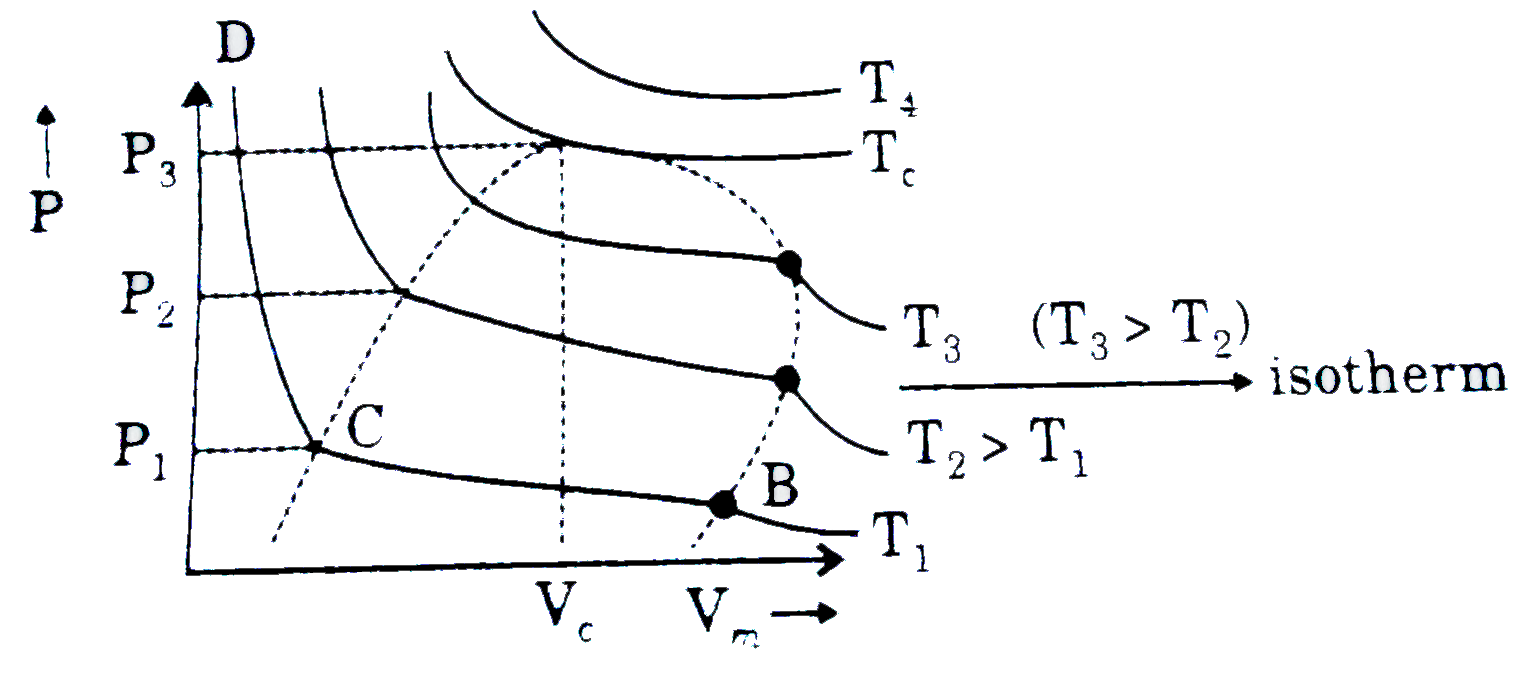
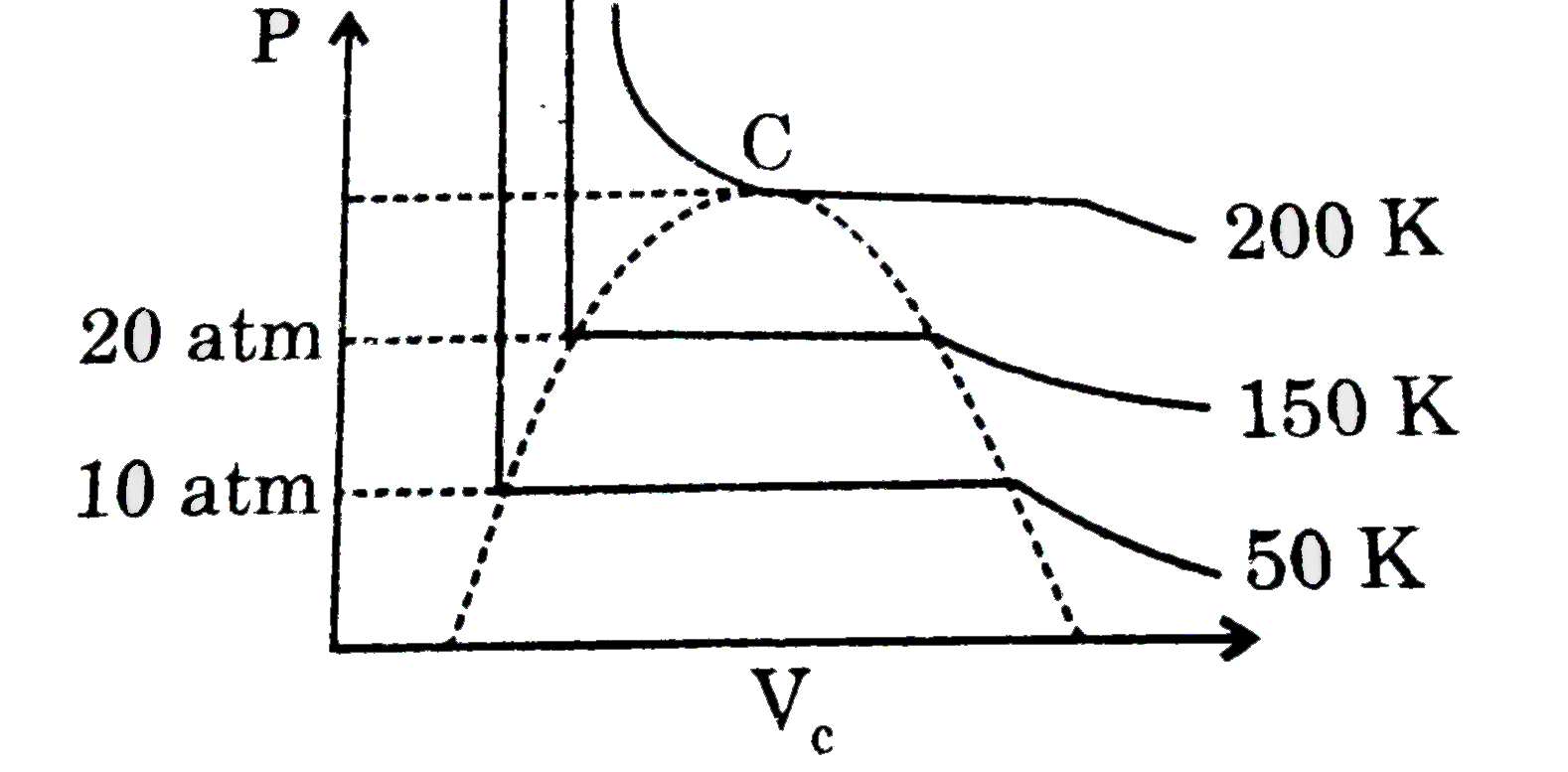
- One of the important approach of the study of real gases involves the ...
Text Solution
|
- When perssure is increase at constant temperature, volume of gas decre...
Text Solution
|
- When perssure is increase at constant temperature, volume of gas decre...
Text Solution
|
- In the photoelectric effect the eletrons are emiited intantaneously fr...
Text Solution
|
- In the photoelectric effect the eletrons are emiited intantaneously fr...
Text Solution
|
- Effect of temperature on the equilibrium process analysed by using the...
Text Solution
|
- Effect of temperature on the equilibrium process analysed by using the...
Text Solution
|
- The variation of rate constant with temperature can be explained by Ar...
Text Solution
|
- The variation of rate constant with temperature can be explained by Ar...
Text Solution
|
- If an element can exist in several oxidation states, it is convenient ...
Text Solution
|
- If an element can exist in several oxidation states, it is convenient ...
Text Solution
|
- If an element can exist in several oxidation states, it is convenient ...
Text Solution
|
- If an element can exist in several oxidation states, it is convenient ...
Text Solution
|
- The principle on conductometric titration is based in the fact that du...
Text Solution
|
- The principle on conductometric titration is based in the fact that du...
Text Solution
|
- Titration are one of the methods we can use to discover the precise co...
Text Solution
|
- Titration are one of the methods we can use to discover the precise co...
Text Solution
|
- The speed of a molecule of a gas changes continuously as a result of c...
Text Solution
|
- The speed of a molecule of a gas changes continuously as a result of c...
Text Solution
|
- Concentrations measured as a function of time when gaseous N(2)O(5) at...
Text Solution
|