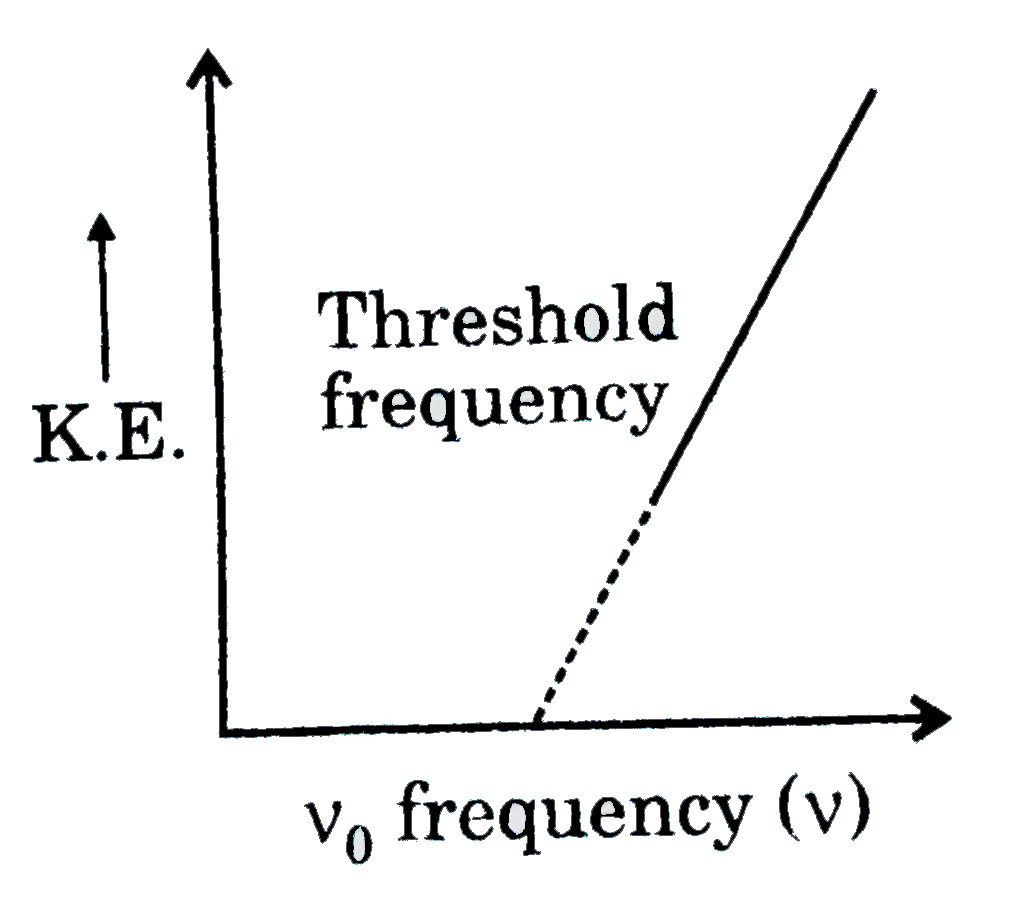In the photoelectric effect the eletrons are emiited intantaneously from a given matal plate when it is irradiated with radiation of frequency equal to or greater then some minimum ferquency, is called the threshold frequency.According to Planck's idea, light may be considered to be made up discrete particles called photons.Each photon carries energy equal ti hv.When this photon cllides with the electron of the metal, the electron acquires energy of the emitted electron is given by :
`hv=K.E_("maximum")+PE=(1)/(2)m u^(2)+PE`
If the incident rediation is of threshold frequency the electron will be emitted without any kinetic energy
i.e `hv_(0)`
`:.(1)/(2)m u^(2)=hv-hv_(0)`
A plot of kinetic energy of the emitted electron versus frequency of the incident radiation yields a straight line given as :
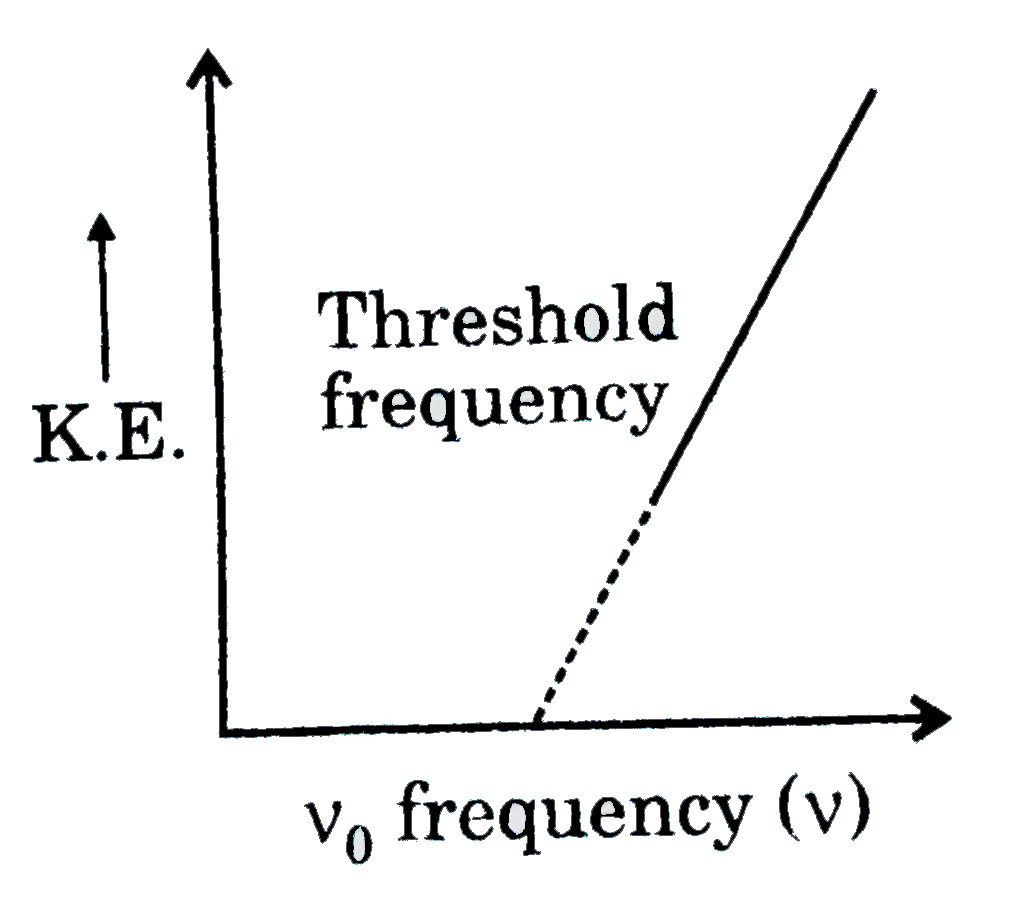
A beam of white light is dispersed into its wavelenght components of potassium metal. What of the electron emitted by the different light component ?