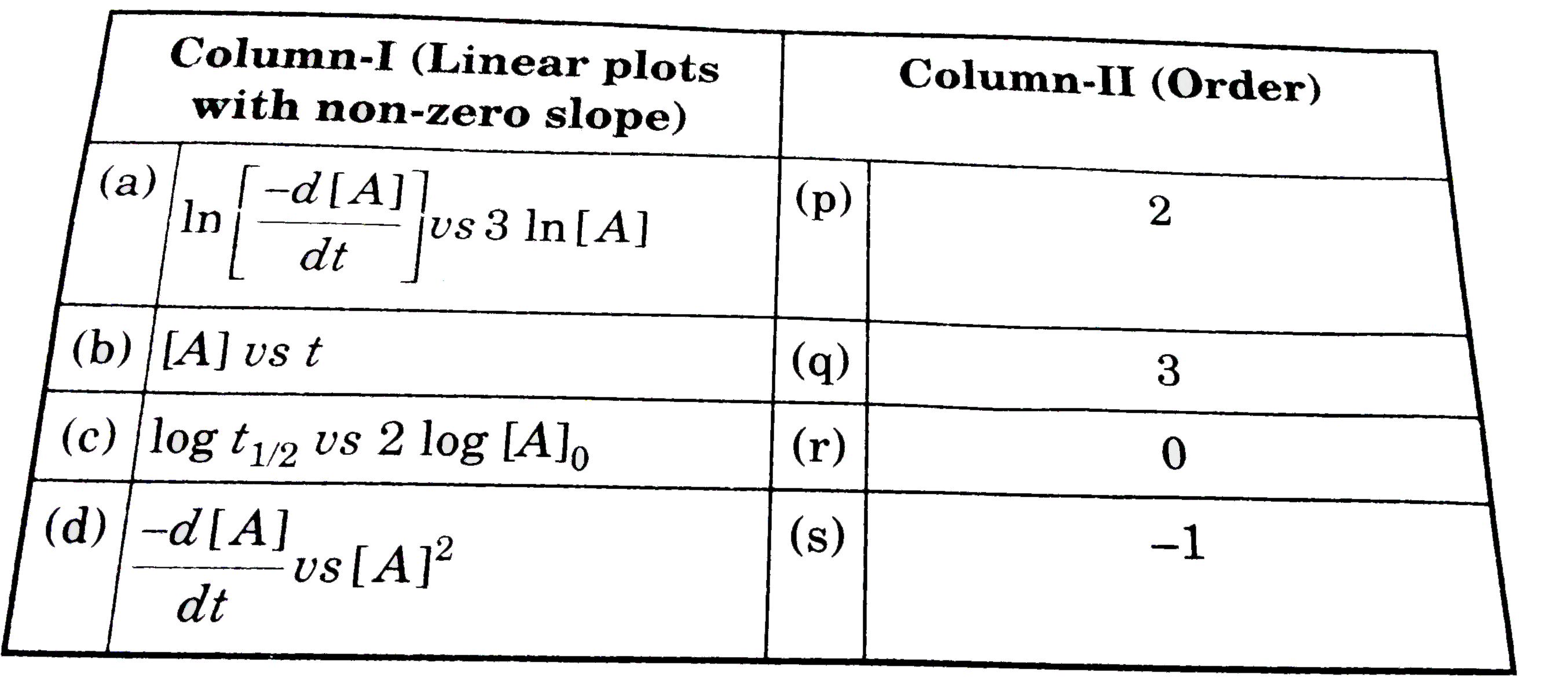Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-GRAPHICAL INTERPRETATION-Match The Column Type
- Match the following columns
Text Solution
|
- For a certain van der Waal's gas, critical temperature is-243^(@)C. Ma...
Text Solution
|
- Match the graphs given in column-I to the parameters and conditions. ...
Text Solution
|
- Match the following columns
Text Solution
|
- The following graphs are plotted for an ideal gas taken from state A t...
Text Solution
|
Text Solution
|
- Match the following columns
Text Solution
|
- In each situation of column-I a process ABC is given for fixed amount ...
Text Solution
|
- [A] = reactant concentration at time 't', k = rate constant
Text Solution
|
- The figures given below depict different processes for a given amount...
Text Solution
|
- Match the following columns
Text Solution
|
- One mole of an ideal gas going from state-A to state-B through differe...
Text Solution
|
- R(r) is the radial part of the wave function and r is the distance of ...
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following Columns
Text Solution
|