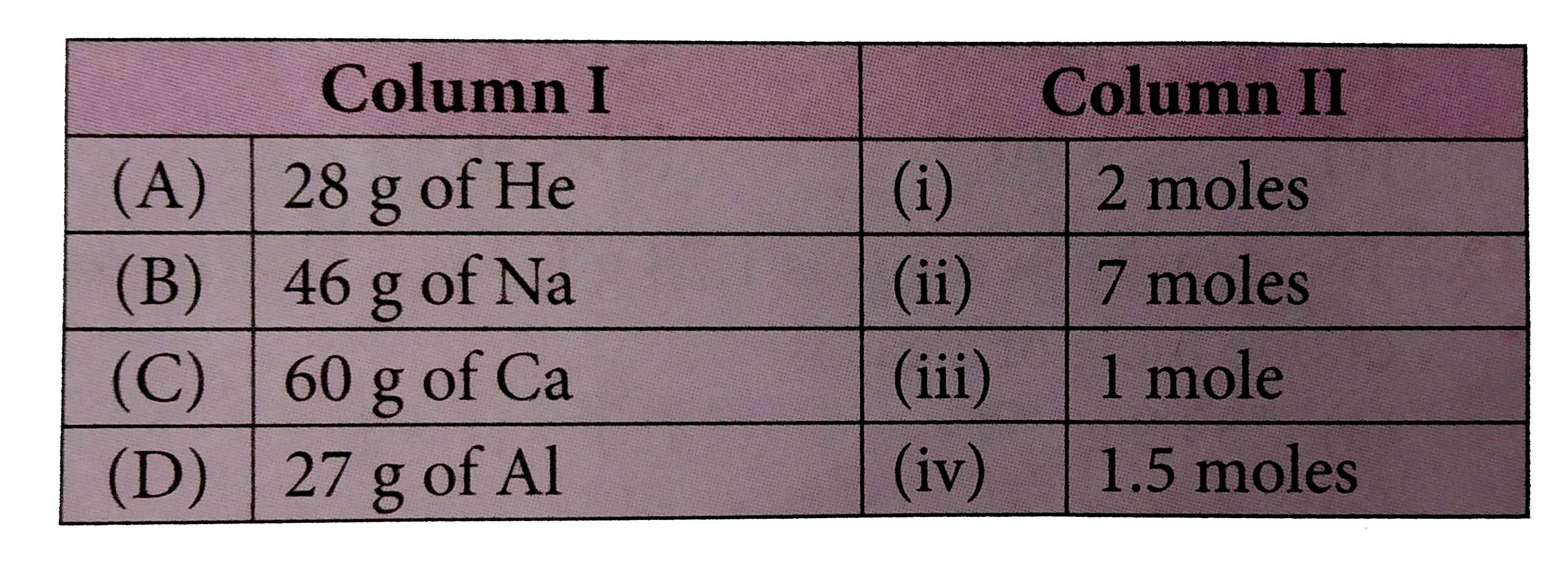
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MARVEL PUBLICATION-BASIC CONCEPT OF CHEMISTRY-TEST YOUR GRASP
- Match the mass of elements given in coloumn I with the no. of moles gi...
Text Solution
|
- Which of the following phrases would be incorrect to use ?
Text Solution
|
- The formula of barium tetrafluorobromate (III) will be
Text Solution
|
- Which of the following is an element ?
Text Solution
|
- The ratio of mass of 1 mole of sulphur and 10^(23) atoms of sulphur is...
Text Solution
|
- Which of the following has maximum number of molecules ? (C = 12, O = ...
Text Solution
|
- Which of the following weighs the maximum ? (O = 16)
Text Solution
|
- If weight of one drop of H2O2 is 3.4xx10^(-5) kg. The number of hydrog...
Text Solution
|
- The number of potassium atoms present in 1 equivalent of KMnO4 is .
Text Solution
|
- Two containers P and Q of equal volume (1 litre each) contain 6 g of O...
Text Solution
|
- 1 amu is equal to
Text Solution
|
- Which of the following contains maximum number of atoms ?
Text Solution
|
- 3 g of an oxide of a metal is converted completely to 5 g chloride. Eq...
Text Solution
|
- The simplest formula of a compound containing 50% of element X (atomic...
Text Solution
|
- 100 cm^(3) of 0.1 N HCl is mixed with 100 cm^(3) of 0.2 N NaOH solutio...
Text Solution
|
- The number of molecules in 16 g of methane is
Text Solution
|
- A molal solution is one that contains 1 mol of a solute dissolved in
Text Solution
|
- How much of NaOH is reuired to neutralise 1500 cm^(3) of 0.1 N HCl (Na...
Text Solution
|
- Which has maximum number of molecules?
Text Solution
|
- 30g Mg and 30g O(2) are reacted and the residual mixture contains:
Text Solution
|
- In the chemical reaction, K2 Cr2 O7 + xH2 SO4 + ySO2 rarr K2SO4 + Cr2(...
Text Solution
|