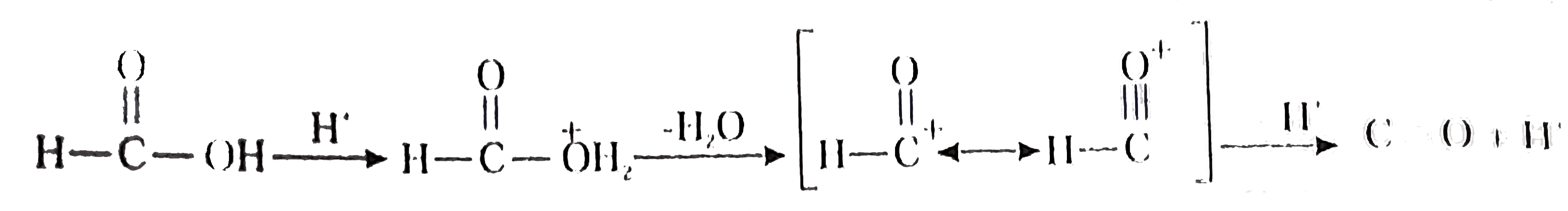A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-CARBOXYLIC ACID-All Questions
- Which of the following will undergo acetylation?
Text Solution
|
- In above reaction, the type of intermediates formed are
Text Solution
|
- Methanoic acid, the first member of carboxylic acid series, when warme...
Text Solution
|
- Methanoic acid, the first member of carboxylic acid series, when warme...
Text Solution
|
- Methanoic acid, the first member of carboxylic acid series, when warme...
Text Solution
|
- Methanoic acid, the first member of carboxylic acid series, when warme...
Text Solution
|
- Consider the pathway of a well known organic reaction For the con...
Text Solution
|
- Consider the pathway of a well known organic reaction Which step ...
Text Solution
|
- Match the column {:("Column I (Acid)","Column II " (pK(a) "Value"))...
Text Solution
|
- Match the column {:("Column I","Column II"),"(A) Acetic acid","(p) d...
Text Solution
|
Text Solution
|
- Statement 1: Carboxylic acids do not give characterstic reactions of c...
Text Solution
|
- Statement 1 : Carboxylic acids have higher boiling points than alkanes...
Text Solution
|
- Statement 1 : Pure acetic acid can be converted into ice like solid ca...
Text Solution
|
- Statement 1 : Lower acids on reacting with strong electropositive meta...
Text Solution
|
- consider the reaction CH(3)OH+CO overset("Cobalt carbonyl")underset...
Text Solution
|
- Consider What is the value of n?
Text Solution
|
- A dicarboxylic acid of the form does not undergo decarboxylation...
Text Solution
|
- Find the total number of products obtained when Undergoes decarbo...
Text Solution
|
- Find the total number of different products obtained when is heat...
Text Solution
|