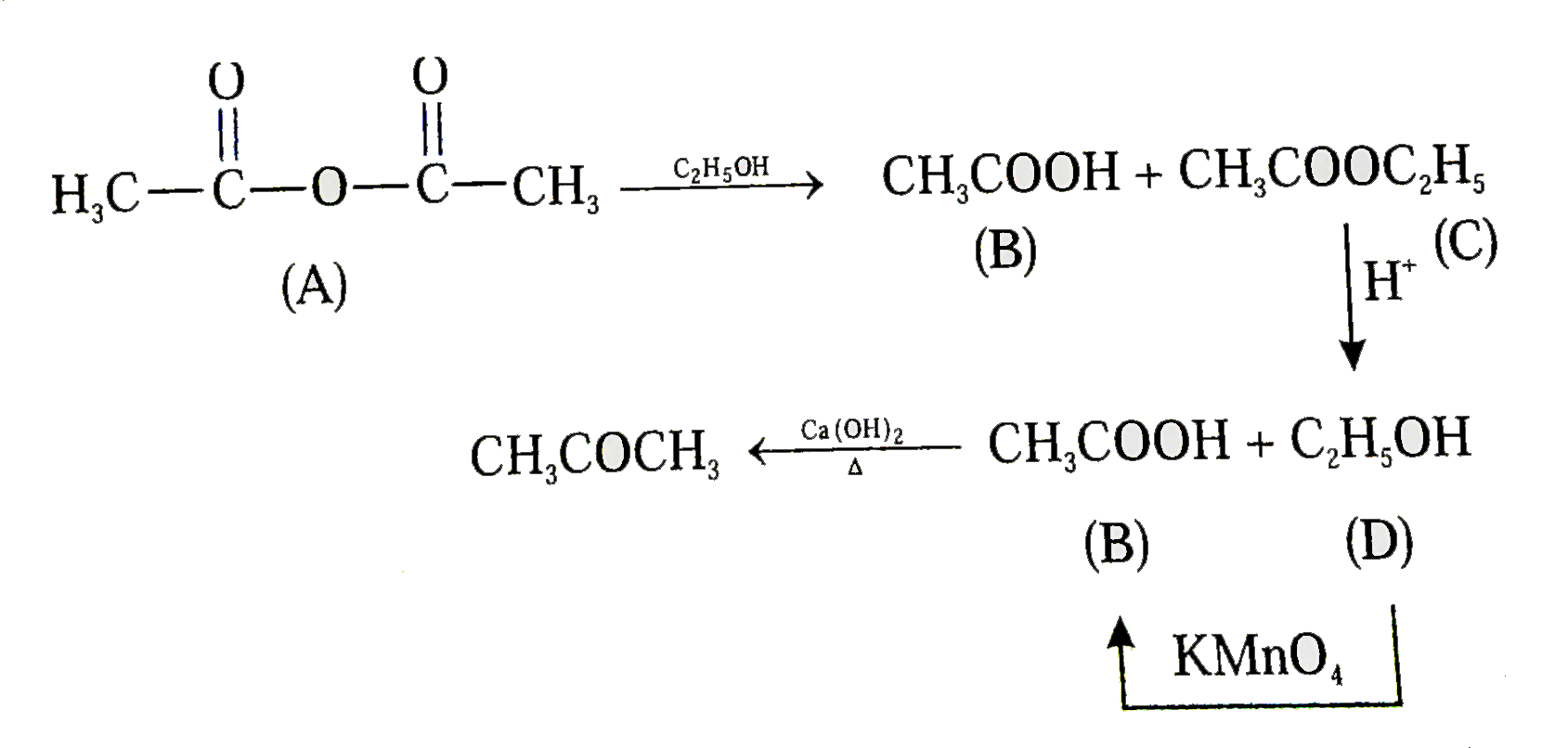
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-CARBOXYLIC ACID-All Questions
- A sweet smelling liquid A has molecular formula C5 H(10) O2 A does not...
Text Solution
|
- An organic compound (A) on treatment with acetic acid in the presence ...
Text Solution
|
- An organic compound (A) on treatment with ethyl alcohol gives a carbox...
Text Solution
|
- A neutral liquid of formula C7 H(14) O2 is hydrolysed to an acid (A), ...
Text Solution
|
- Compound A, C4 H8 O2 has the following properties. (i) it reacts with ...
Text Solution
|
- Identify X, Y and Z in the following synthetic scheme and write their ...
Text Solution
|
- An organic compound (A) (C5 H8 O3) on heating with soda lime gives (B)...
Text Solution
|
- The sodium salt of a carboxylic acid (A) was produced by passing a gas...
Text Solution
|
- Chlorination of toluene in the presence of light and heat followed by ...
Text Solution
|
- Hydrogenation of benzoyl chloride in the presence of Pd on BaSO4 gives
Text Solution
|
- The organic product formed in the reaction C(6)H(5)CO OH overset(1. L...
Text Solution
|
- When propionic acid is treated with aqueous sodium bicarbonate, CO2 is...
Text Solution
|
- Benzoyl chloride is prepared from benzoic acid by
Text Solution
|
- The product of acid hydrolysis of P and Q can be distinguished by
Text Solution
|
- An enantiomerically pure acid is treated with racemic mixture of an al...
Text Solution
|
- Which of the following reactants on reaction with conc. NaOH followed ...
Text Solution
|
- In the following reaction sequence, products I, J, and L are formed. K...
Text Solution
|
- In the following reaction sequence, products I, J, and L are formed. K...
Text Solution
|
- n the following sequence, product I,J and L are formedK represents a r...
Text Solution
|
- Acetic acid does not undergo haloform reaction. Acetic acid has no a...
Text Solution
|