Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NN GHOSH-FIRST LAW OF THERMODYNAMICS-All Questions
- In the determination of J buy Joule's experiment, the weights of mass ...
Text Solution
|
- An immersion type electric heater of 250 watts is immersed in 5 kg of...
Text Solution
|
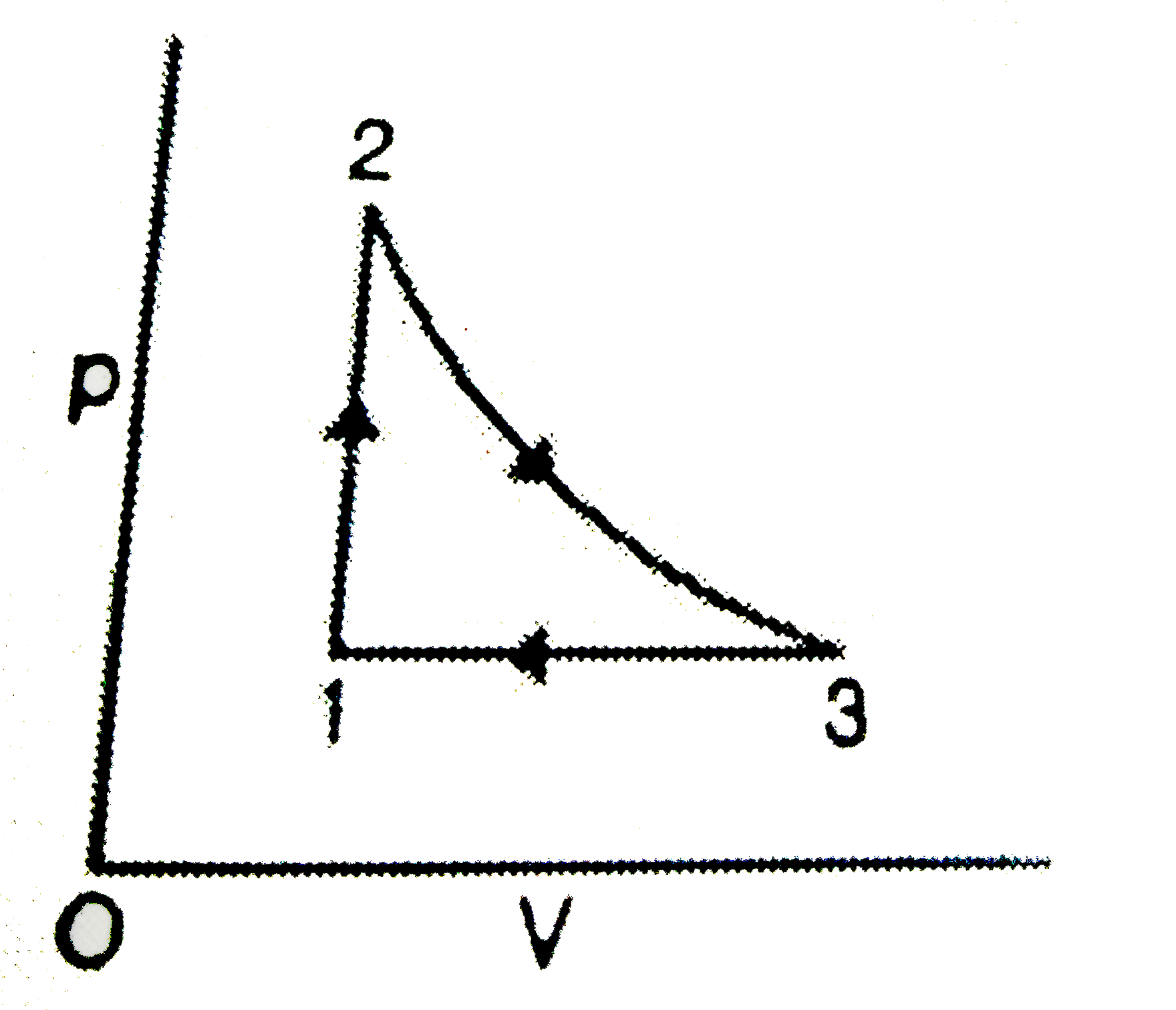
- A cyclic process for an ideal monatomic gas (C(v)=12.5Jmol^(-1)K^(-1))...
Text Solution
|
- Find the internal energy of air in a room of volume 40m^(3) at 1 stand...
Text Solution
|
- A saturated water vaporu (M=18) is contained in a vessel fitted with a...
Text Solution
|
- Water of mass m=1kg and M (mol mass) =18 turns completely into saturat...
Text Solution
|
- The height of the Niagara Falls is 50m.Calculate the difference betwee...
Text Solution
|
- If a slice of bread can supply 100 kilocalorie of heat to a man, how m...
Text Solution
|
- How much work is needed to convert 5g of ice at -3^(@)C to steam at 10...
Text Solution
|
- A tube of length 2m containing a little mercury and closed at both end...
Text Solution
|
- How much work is done agaist uniform pressure when 1g of water at 100^...
Text Solution
|
- A meteorite weighing 2000kg enters the earth's atmosphere with a velo...
Text Solution
|
- If a lead bullet be suddenly stopoed and all its energy be used to hea...
Text Solution
|
- From what height must a block of ice fall to just melt by the impact a...
Text Solution
|
- With what velocity must a lead bullet at 50^(@)C strike against an obs...
Text Solution
|
- A block of ice is dropped into a well of water, both ice and water be...
Text Solution
|
- A hole is drilled into a block of lead of mass 10kg by a driller. The ...
Text Solution
|
- A bullet of lead melts when stopped by obstacle. Assuming that 25 per ...
Text Solution
|
- A thermally insulated vessel containing a gas, whose molar mas is equa...
Text Solution
|
- Gaseous hydrogen initially contained under standard conditions in a se...
Text Solution
|