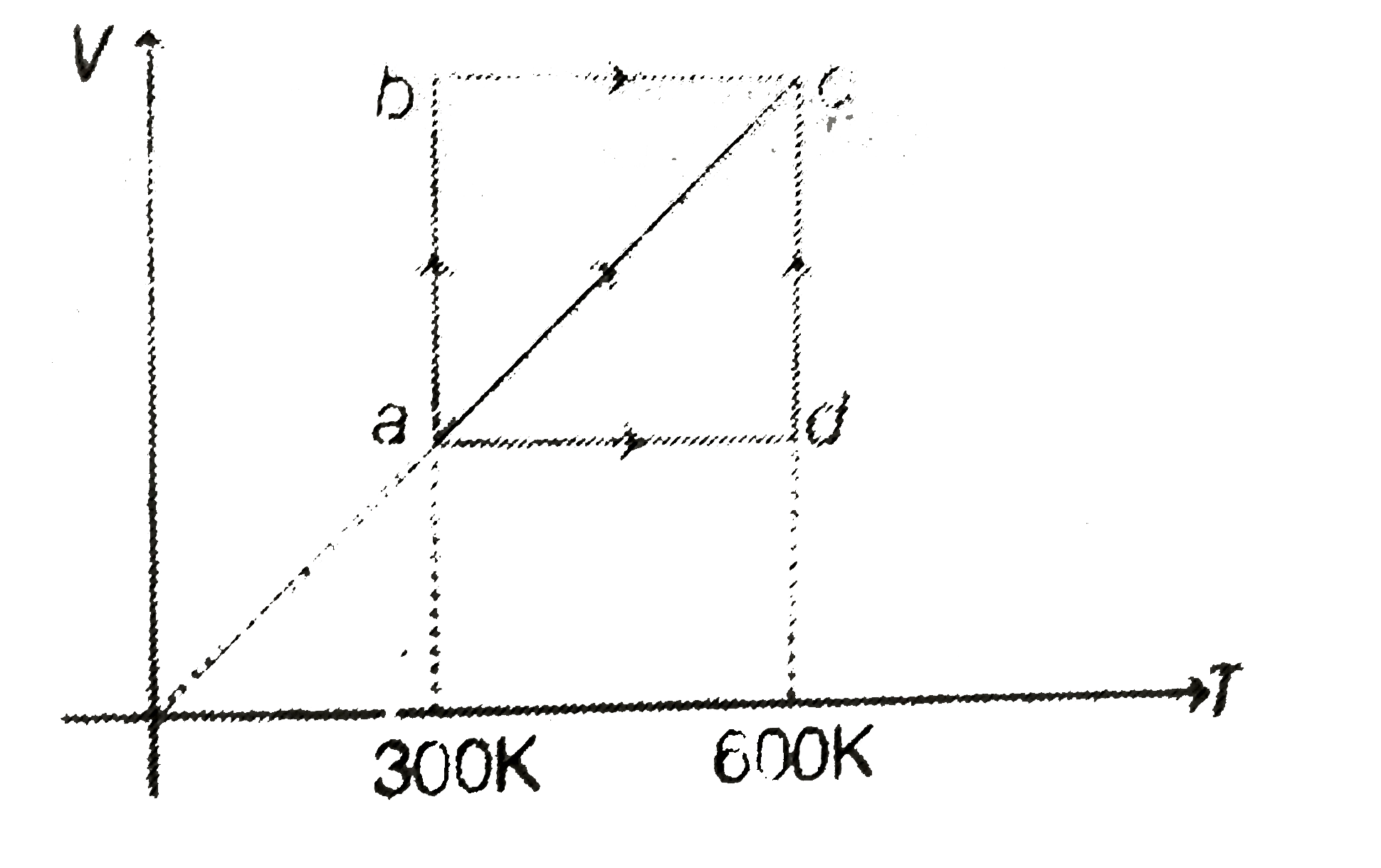
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DC PANDEY-CURRENT ELECTRICITY-All Questions
- graph of an ideal gas is as shown in figure. Heat is supplied to ...
Text Solution
|
- Two moles of a monoatomic gas are taken from a to c, via three paths a...
Text Solution
|
- Two moles of a monoatomic gas are taken from a to c, via three paths a...
Text Solution
|
- The internal energy of a gas is given by U = 3 pV. It expands from V(0...
Text Solution
|
- The internal energy of a gas is given by U = 3 pV. It expands from V(0...
Text Solution
|
- A certain amount of ice is supplied heat at a constant rate for 7 min....
Text Solution
|
- A certain amount of ice is supplied heat at a constant rate for 7 min....
Text Solution
|
- An ideal diatomic gas with C(V)=(5R)/(2) occupies a volume V(1) at a p...
Text Solution
|
- An ideal diatomic gas with C(V)=(5R)/(2) occupies a volume V(1) at a p...
Text Solution
|
- When a system is taken from state 'a' to state 'b' along the path 'acd...
Text Solution
|
- When a system is taken from state 'a' to state 'b' along the path 'acb...
Text Solution
|
- When a system is taken from state 'a' to state 'b' along the path 'acb...
Text Solution
|
- When a system is taken from state 'a' to state 'b' along the path 'acb...
Text Solution
|
- Two rods A and B of same cross-sectional area A and length l are conne...
Text Solution
|
- Two rods A and B of same cross-sectional area A and length l are conne...
Text Solution
|
- Two rods A and B of same cross-sectional area A and length l are conne...
Text Solution
|
- A sample of ideal gas is taken through the cyclic process shown in the...
Text Solution
|
- A sample of ideal gas is taken through the cyclic process shown in the...
Text Solution
|
- A sample of ideal gas is taken through the cyclic process shown in the...
Text Solution
|
- One mole of an ideal monoatomic has is taken through cyclic process AB...
Text Solution
|