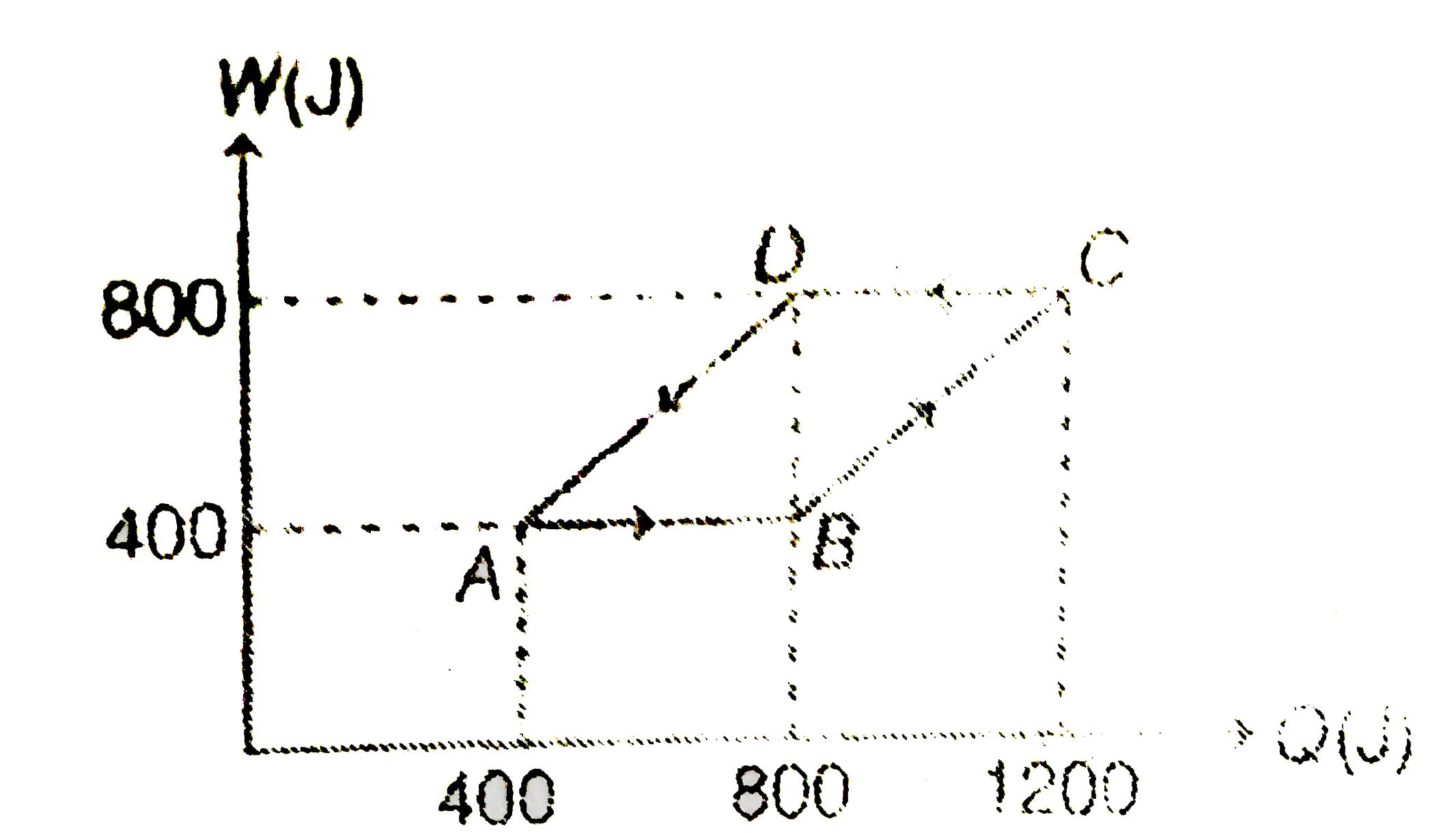
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DC PANDEY-CURRENT ELECTRICITY-All Questions
- One mole of an ideal monotomic gas undergoes a linear process from A t...
Text Solution
|
- A sample of mono-atomic ideal gas is taken through a cyclic process AB...
Text Solution
|
- A sample of mono-atomic ideal gas is taken through a cyclic process AB...
Text Solution
|
- A metal block is placed in a room which is at 10^(@)C for long time. N...
Text Solution
|
- A metal block is placed in a room which is at 10^(@)C for long time. N...
Text Solution
|
- Four moles of an ideal gas is initially in a state A having pressure 2...
Text Solution
|
- Four moles of an ideal gas is initially in a state A having pressure 2...
Text Solution
|
- Two moles of a diatomic gas are carried trhough the cycle ABCDA as sho...
Text Solution
|
- Two moles of a diatomic gas are carried trhough the cycle ABCDA as sho...
Text Solution
|
- Two moles of a diatomic gas are carried trhough the cycle ABCDA as sho...
Text Solution
|
- 2 moles of an ideal monoatomic gas undergoes a cyclic process ABCA as ...
Text Solution
|
- 2 moles of an ideal monoatomic gas undergoes a cyclic process ABCA as ...
Text Solution
|
- One mole of helium gas follows the cycle 1-2-3-1 shown in the diagram....
Text Solution
|
- One mole of helium gas follows the cycle 1-2-3-1 shown in the diagram....
Text Solution
|
- Match the following
Text Solution
|
- Match the following.
Text Solution
|
- Match the following.
Text Solution
|
- Match the following two tables.
Text Solution
|
- The energy of the rotational motion of the molecules in n moles of nit...
Text Solution
|
- Two moles of a diatomic ideal gas is taken through pT= constant. Its t...
Text Solution
|