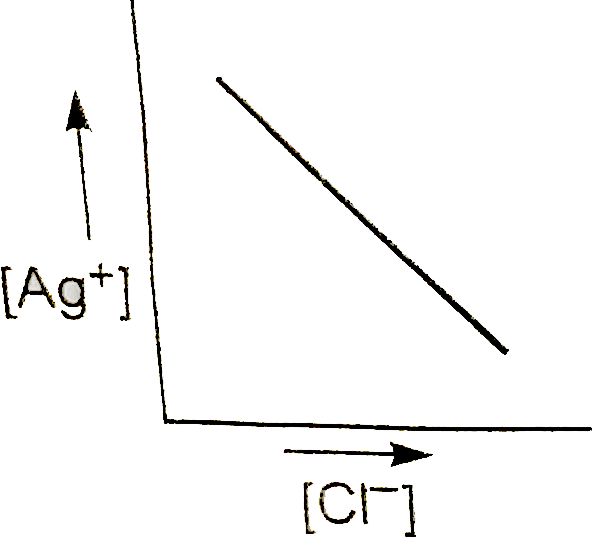
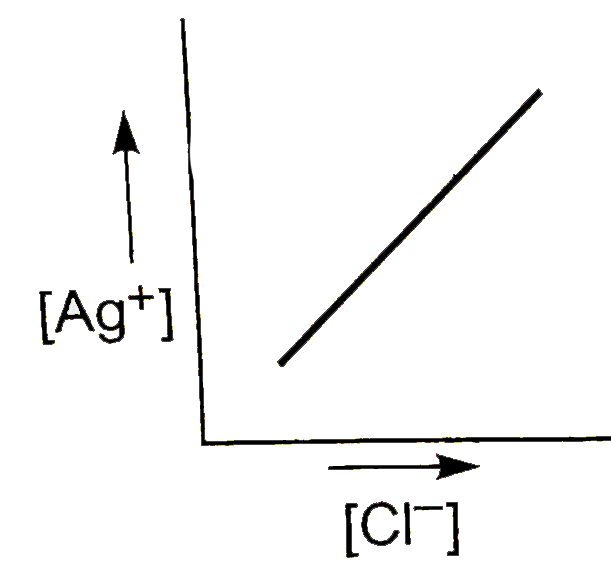
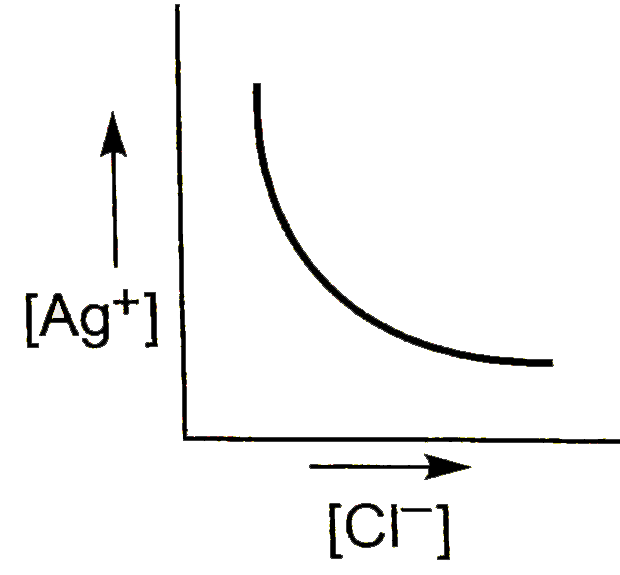
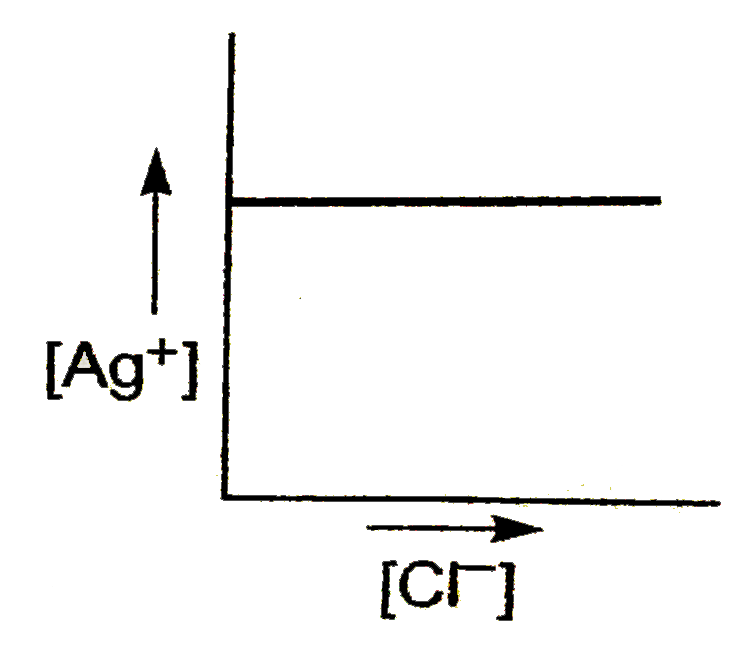
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI-IONIC EEQUILIBRIUM-Exercise
- A 1 litre solution containing NH(4)Cl and NH(4)OH has hydroxide ion io...
Text Solution
|
- 150 mL of 0.0008 M ammonium sulphate is mixed with 50 mL of 0.04 M cal...
Text Solution
|
- In a saturated solution of AgCl, NaCl is added gradually. The concentr...
Text Solution
|
- K(sp) of AgCl is 1xx10^(-10). Its solubility in 0.1 M KNO(3) will be :
Text Solution
|
- 50mL of a solution containing 10^(-3) mole of Ag^(+) is mixed with 5...
Text Solution
|
- At a certain temperature, the solubility of the salt A(x)B(y) is S mol...
Text Solution
|
- What is the molarity of a saturated solution of CaCO(3)? (K(sp)=2.8xx1...
Text Solution
|
- K(sp) of Zr(3)(PO(4))(4) in terms of solubility (S) is :
Text Solution
|
- The solubility of electrolytes MX(1),MX(2) and MX(3) is 1xx10^(-3) mol...
Text Solution
|
- A saturated solution of Ca(3)(PO(4))(2) has [Ca^(2+)]=2xx10^(-8) M and...
Text Solution
|
- Which of the following is most soluble in water?
Text Solution
|
- Silver ions are added to a solution with [Br^(-)]=[Cl^(-)]=[CO(3)^(2-)...
Text Solution
|
- The solubility of different springly soluble salts are given as under ...
Text Solution
|
- If K(sp) for HgSO(4) is 6.4xx10^(-5), then solubility of this substanc...
Text Solution
|
- The solubility of Ba(3)(AsO(4))(2)(formula mass=690) is 6.9xx10^(-2) g...
Text Solution
|
- The solubility of AgBrO(3) (formula mass=236) is 0.0072 g in 1000 mL. ...
Text Solution
|
- The solubility of PbF(2) (formula mass =245) is 0.46 g/L. What is the ...
Text Solution
|
- How many grams of MgC(2)O(4) (formula mass =122) will dissolve in 1.5 ...
Text Solution
|
- What is the molarity of F^(-) ions in a saturated solution of BaF(2)? ...
Text Solution
|
- What is the molarity of F^(-) in a saturated solution of In F(3)?(K(sp...
Text Solution
|