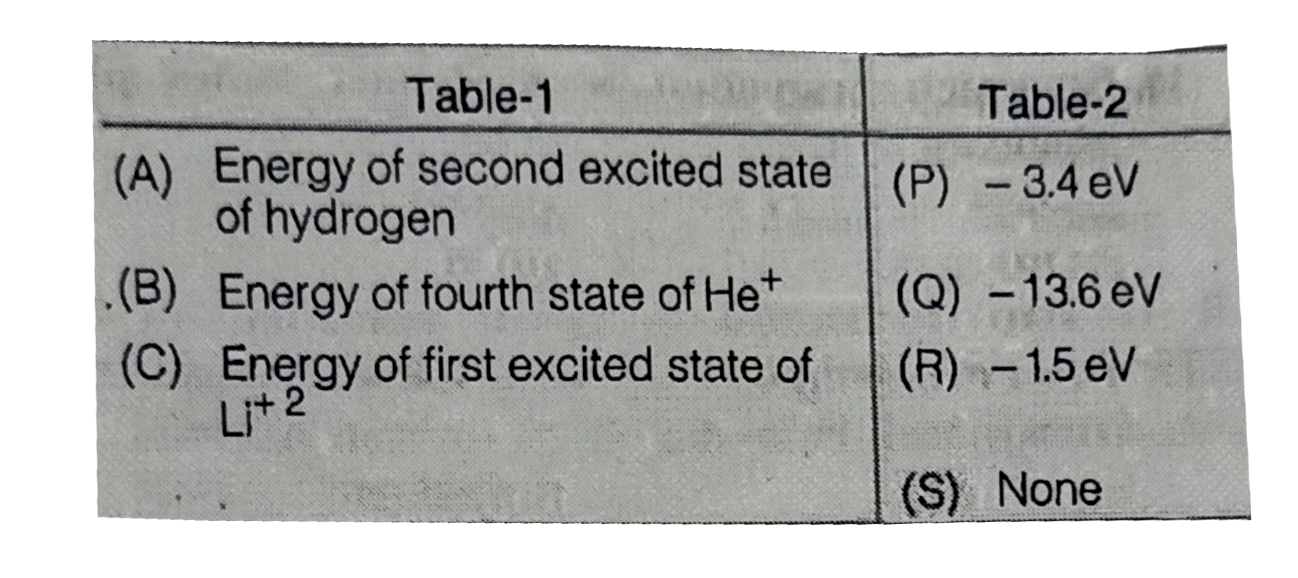
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DC PANDEY-MODERN PHYSICS-Metch the column
- sqrt(v) versus Z graph for dcharacteristic X-rays is as shown in figur...
Text Solution
|
- Regarding trasnsition of electrons match the following table
Text Solution
|
- Match the following table.
Text Solution
|
- Excitions energy of hydrogen atom is 13.6 eV mathc the following
Text Solution
|
- In a nuclear reactor mathc the following .
Text Solution
|
- In each situationof Table-1 a physicial quantity relate to orbiting el...
Text Solution
|