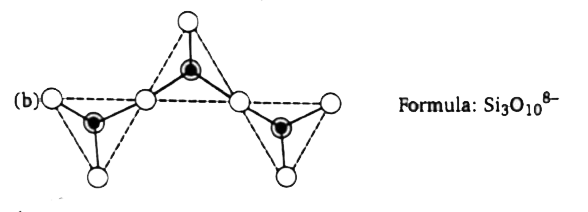
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VK JAISWAL-p-BLOCK ELEMENTS-SUBJECTIVE PROBLEMS
- The silicate anion in the mineral kinoite is a chain of three SiO(4) t...
Text Solution
|
- In phosphorus acid, If X is number of non-bonding electron pairs. Y is...
Text Solution
|
- Consider the following oxyanions: PO(4)^(3-),P(2)O(6)^(4-),SO(4)^(2-...
Text Solution
|
- For oxyacid HClO(x), fi x=y=z (x,y and z are natural numbers), then ca...
Text Solution
|
- Consider the following representation of oxy-acid, H(n(1))S(2)O(n(2)),...
Text Solution
|
- total number of molecule which hydrolysed at room temperature and hybr...
Text Solution
|
- The difference between total number of lone pairs and total number of ...
Text Solution
|
- Calculate vlaue of |x+y-z| for the followng sillicate [Si(x)O(y+z)]^(z...
Text Solution
|
- The general formula of polythionate ion is S(n+2)O(6)^(2-). If average...
Text Solution
|
- total number of Boron atoms in anionic part of borax which participate...
Text Solution
|
- Choose total number of correct reaction. (i) When CuSO(4) solution r...
Text Solution
|
- Consider the following orders: (1) H(2)SO(4) gt H(2)SO(3):boiling po...
Text Solution
|
- How many monovalent oxygen atoms are preset in the mineral kinoite (ov...
Text Solution
|
- How many moles off given compound are decomposed in the following deco...
Text Solution
|
- How many moles of NaOH are required to react with one mole of solid N(...
Text Solution
|
- How many moles of hypophophorous acid are involved in its thermal deco...
Text Solution
|
- Consider the structure of Al(2)Me(6) compound and find the value of (x...
Text Solution
|
- Sum of oxidation state of nitrogen atom in hyponitrous acid, nitric ac...
Text Solution
|
- Find the value of x in the tremolite abestos: Ca(2)Mg(x)(Si(4)O(11)...
Text Solution
|
- Consider the following silicates (a) BaTi(Si(2)O(9)) (b) ZnCa(2)Si...
Text Solution
|
- Atomicity of white or yellow phosphorus is 4 annd it is represented as...
Text Solution
|