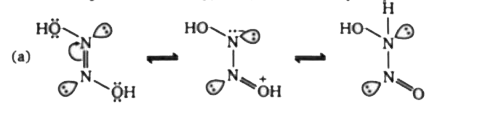
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
p-BLOCK ELEMENTS
VK JAISWAL|Exercise ONE OR MORE ANSWERS IS/ARE CORRECT|97 Videosp-BLOCK ELEMENTS
VK JAISWAL|Exercise MATCH THE COLUMN|24 Videosp-BLOCK ELEMENTS
VK JAISWAL|Exercise LEVEL 2|97 VideosMETALLURGY
VK JAISWAL|Exercise ASSERTION-REASON TYPE QUESTIONS|30 VideosPERIODIC PROPERTIES
VK JAISWAL|Exercise ASSERTION-REASON TYPE QUESTIONS|35 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL-p-BLOCK ELEMENTS-LEVEL 3 (PASSAGE TYPE)
- Each oxy-acid contains at least one X-OH unit (X is non-metal). The H-...
Text Solution
|
- Each oxy-acid contains at least one X-OH unit (X is non-metal). The H-...
Text Solution
|
- Each oxy-acid contains at least one X-OH unit (X is non-metal). The H-...
Text Solution
|
- Formation of a bridge bond is best explained by molecular orbital theo...
Text Solution
|
- Formation of a bridge bond is best explained by molecular orbital theo...
Text Solution
|
- Formation of a bridge bond is best explained by molecular orbital theo...
Text Solution
|
- (i) P+C("carbon")+Cl(2) to Q+CO uaarr (ii) Q+H(2)O to R+HCl (iii) ...
Text Solution
|
- (i) P+C("carbon")+Cl(2) to Q+CO uaarr (ii) Q+H(2)O to R+HCl (iii) ...
Text Solution
|
- (i) P+C("carbon")+Cl(2) to Q+CO uaarr (ii) Q+H(2)O to R+HCl (iii) ...
Text Solution
|
- Q. Compound (D)+I^(-)+H^(-) to Gas Evolved gas is similar to:
Text Solution
|
- Q. Yellow ppt. of compound (I) is insoluble in:
Text Solution
|
- Q. type of hybridization of central atomo of gas (B) is:
Text Solution
|
- The following flow diagram represent the industrial preparation of nit...
Text Solution
|
- The following flow diagram represent the industrial preparation of nit...
Text Solution
|
- Species having X-O-H linkage (X=non-metal with positive oxidation stat...
Text Solution
|
- Species having X-O-H linkage (X=non-metal with positive oxidation stat...
Text Solution
|
- Species having X-O-H linkage (X=non-metal with positive oxidation stat...
Text Solution
|
- Consider the following sequence of reactions, if A is sulphuric acid, ...
Text Solution
|
- Consider the following sequence of reactions, if A is sulphuric acid, ...
Text Solution
|
- Consider the following sequence of reactions, if A is sulphuric acid, ...
Text Solution
|