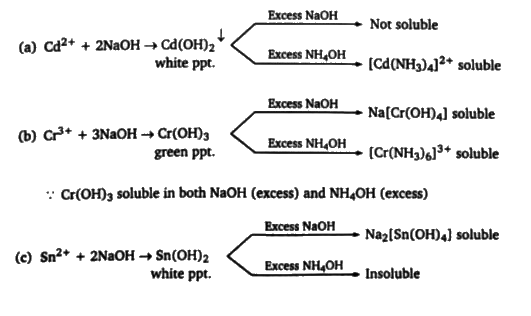
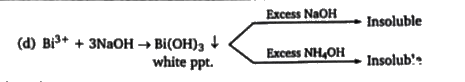A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
QUALITATIVE INORGANIC ANALYSIS
VK JAISWAL|Exercise MATCH THE COLUMN|2 VideosQUALITATIVE INORGANIC ANALYSIS
VK JAISWAL|Exercise ASSERTION-REASON TYPE QUESTIONS|18 VideosQUALITATIVE INORGANIC ANALYSIS
VK JAISWAL|Exercise LEVEL 3|72 VideosPERIODIC PROPERTIES
VK JAISWAL|Exercise ASSERTION-REASON TYPE QUESTIONS|35 Videoss-BLOCK ELEMENTS
VK JAISWAL|Exercise SUBJECTIVE PROBLEMS|2 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL-QUALITATIVE INORGANIC ANALYSIS-ONE OR MORE ANSWER IS/ARE CORRECT
- Which of the following substance are blue?
Text Solution
|
- On raction with dilute H(2)SO(4), which of the following salts will gi...
Text Solution
|
- A yellow precipitate is obtained when:
Text Solution
|
- Which of the following ions can be separated by using NH(4)Cl and NH(4...
Text Solution
|
- Which of the following pairs of cations cannot be separated by adding ...
Text Solution
|
- Which of the following mixtures of ions in solution can be separted by...
Text Solution
|
- Which of the following compound are coloured?
Text Solution
|
- Which of the followig mixtures of ions in solubiotn can be separted by...
Text Solution
|
- Which of the following species will be decomposed on acidification?
Text Solution
|
- Which of the following mixtures of ions in solution can be separated b...
Text Solution
|
- Which of the following ions can be separated by using dilute HCl?
Text Solution
|
- Which of the following substance will leave a black residue on strong ...
Text Solution
|
- By which of the following reagents can a sublimate of HgCl(2) be disti...
Text Solution
|
- An aqueous solution is prepared by dissolving a mixture containing ZnC...
Text Solution
|
- An aqueous solution containing S^(2-) ions will not give:
Text Solution
|
- Which of the following statement (s) is (are) correct when a mixture ...
Text Solution
|
- Choose the correct reaction:
Text Solution
|
- Which of the following aqueous solution of cation(s) give(s) white ppt...
Text Solution
|
- Al(2)(SO(4))(3)+NH(4)OH to X Select the correct statement(s) about c...
Text Solution
|
- The evolution of a red-brown gas on heating a salt with K(2)Cr(2)O(7) ...
Text Solution
|