Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-ALKENES-Additional Straight O Q
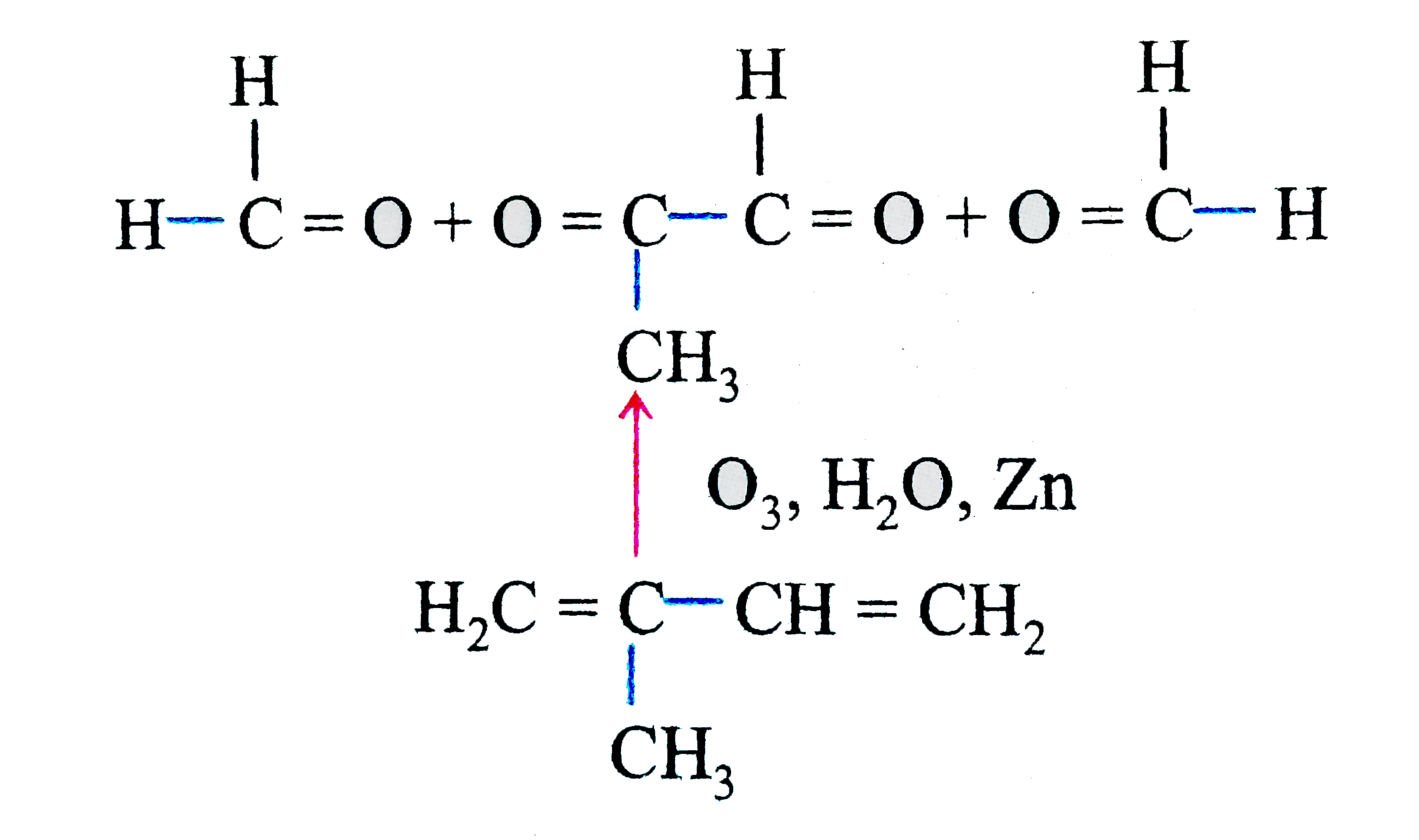
- Isoprene is a diene. How are the positions of double bonds located?
Text Solution
|
- Major product of the reaction
Text Solution
|
- Product (C) of reaction is
Text Solution
|
- Compare rate of dehydration of (i), (ii) and (iii) by conc. H(2)SO(4).
Text Solution
|
- Product (C) is
Text Solution
|
- Product (A) of the above reaction is:
Text Solution
|
- product.
Text Solution
|
Text Solution
|
- Complete the following reaction
Text Solution
|