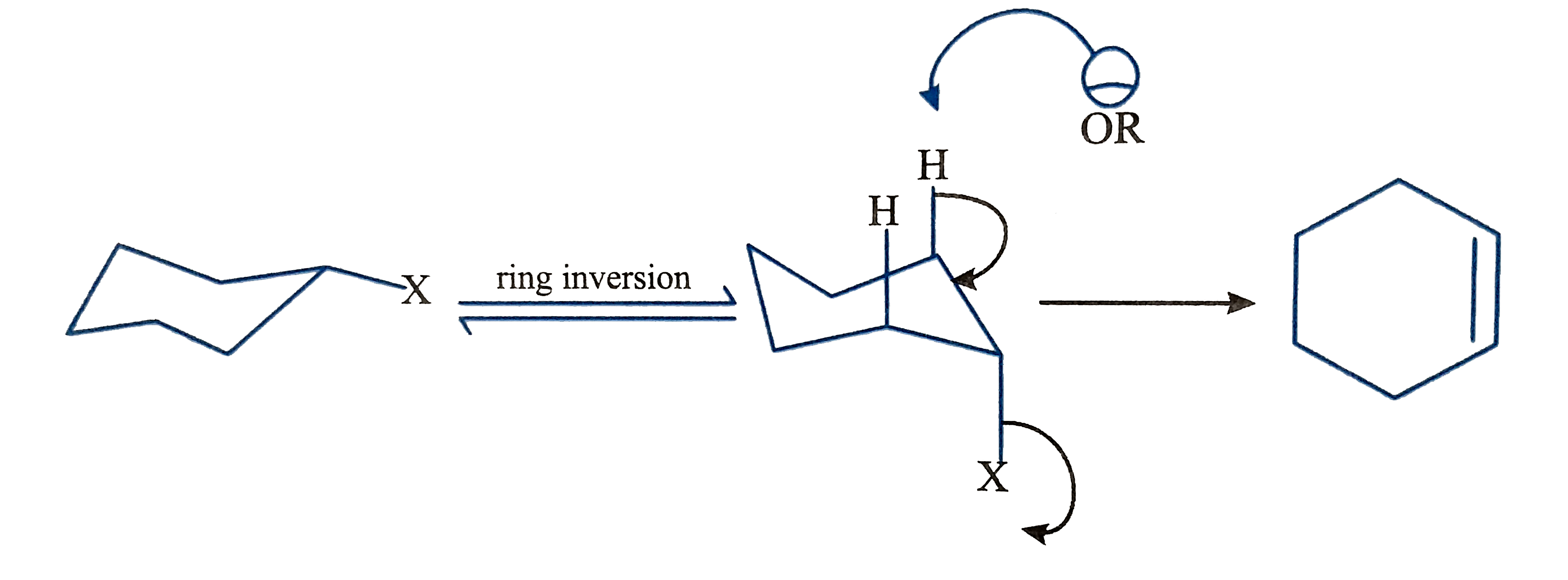
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-ALKENES-level-5 Straight Objective Questions
- Alkenes can be prepared by Cope elimination, hofmann elimination and p...
Text Solution
|
- Alkenes can be prepared by Cope elimination, hofmann elimination and p...
Text Solution
|
- The stereospecificity of the reactions is a very good evidence that E2...
Text Solution
|
- The stereospecificity of the reactions is a very good evidence that E2...
Text Solution
|
- The stereospecificity of the reactions is a very good evidence that E2...
Text Solution
|
- Dehydrtion of alcohol is unimolecular as well as bimolecular reaction....
Text Solution
|
- Dehydrtion of alcohol is unimolecular as well as bimolecular reaction....
Text Solution
|
- Match the column
Text Solution
|
- Match the following
Text Solution
|
- Match each of the compound in column I with the product of reductive o...
Text Solution
|
- Statement-I: Cis-2- butene gives meso compound with Baeyer's reagent. ...
Text Solution
|
- Assertion: Addition of Br(2) to 1-butane gives two optical isomers. ...
Text Solution
|
- Statement-I: Reaction of cold alkaline KMnO(4) with alkenes is regiose...
Text Solution
|
- Statement-I: Alkenes are more reactive than alkynes towards HX. Stat...
Text Solution
|
- Statement-I: Cis-2-butene gives dl-mixture with bromine. Statement-I...
Text Solution
|
- Statement-I: Carbon-carbon double bond length in 2-butene is more than...
Text Solution
|
- Statement I: 1-Butene on reaction with HBr in the presence of a peroxi...
Text Solution
|
- Let us consider the following reaction: CH(2)=CHCH=CH(2)+HBr(leq)ove...
Text Solution
|
- If the following compound is treated with Pd/C in excess of hydrogen g...
Text Solution
|
- When 3-bromo-2,2-dimethyl butane is treated with alcoholic potassiumn ...
Text Solution
|