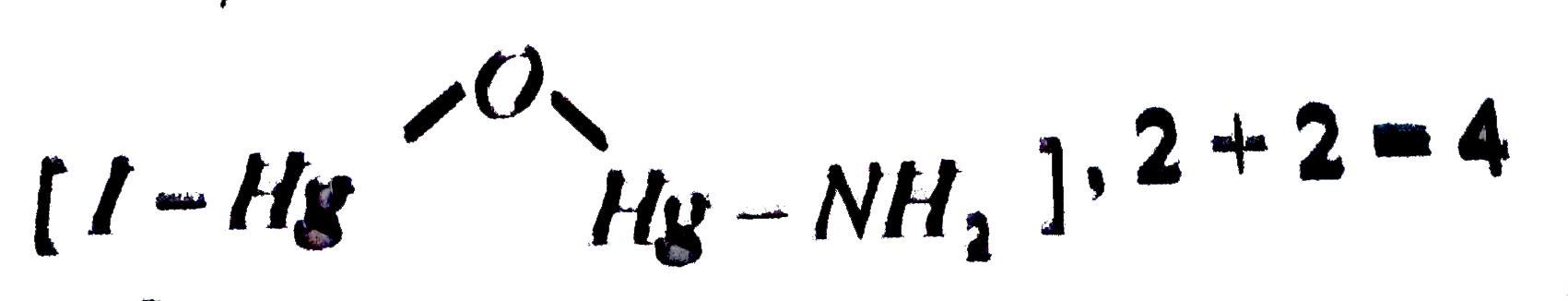Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HALOGEN COMPOUNDS
NARAYNA|Exercise Previous IIT JEE Question|11 VideosHALOGEN COMPOUNDS
NARAYNA|Exercise Passage 4|1 VideosHALOGEN COMPOUNDS
NARAYNA|Exercise Matric Matching questions|21 VideosHALOALKANE AND HALOARENES
NARAYNA|Exercise EXERCISE - 4|59 VideosMETALLURGY
NARAYNA|Exercise STATEMENT TYPE QUESTOINS|20 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-HALOGEN COMPOUNDS-Integer Type Questions
- How many of the following can be oxidized by acid KMnO(4) solution ? ...
Text Solution
|
- Complete the reaction and find out (p+q)=R+4, the value of R is
Text Solution
|
- Cr(2)O(7)^(2-)+xH^(+)+yI^(-)rarr 2Cr^(3+)+I(2)+H(2)O Balance the eeq...
Text Solution
|
- Pb(3)O(4)+underset("Conc.")(xHCl)underset(Delta)(rarr)A+B uarr+H(2)O. ...
Text Solution
|
- Pb(3)O(4)+HNO(3)rarr A+B+H(2)O.A is soluble in water. The oxidation st...
Text Solution
|
- The number of mercury atoms in iodide of Millons base is x and the oxi...
Text Solution
|
- When excess conc. HCl is added to SnCl(4)H(2)[SnCl(x)] is formed. 'x' ...
Text Solution
|
- Number of salts among the following that will give metal on heating ...
Text Solution
|