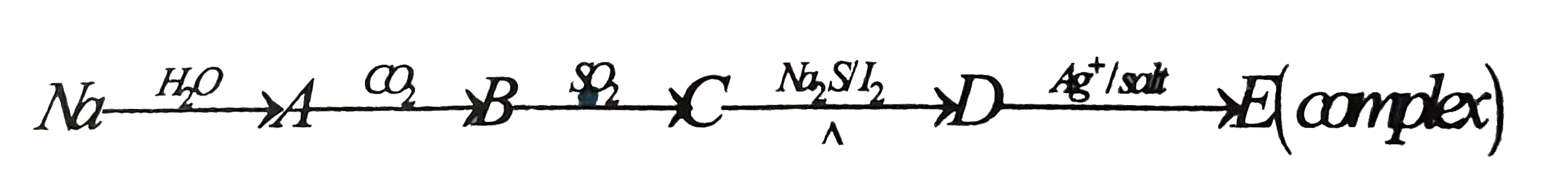A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-S-BLOCK ELEMENTS-Comprehension
- The compound B and C are :
Text Solution
|
- The compound D is
Text Solution
|
- Oxidation number of each 'S' atom in compound D :
Text Solution
|
- Sodium carbonate is generally prepared by a process called ammonia sod...
Text Solution
|
- Sodium carbonate is generally prepared by a process called ammonia sod...
Text Solution
|
- Sodium carbonate is generally prepared by a process called ammonia sod...
Text Solution
|
- Compound A is produced by absorbing dinitrogen trioxide in NA(2)CO(3) ...
Text Solution
|
- Gas B is paramagnetic and support the combustion. Compound B is
Text Solution
|
- D is inert gas, which is also obtined by strongely heating the ammoniu...
Text Solution
|
- V and P can be
Text Solution
|
- Q and S can be
Text Solution
|
- R, u, T can be
Text Solution
|