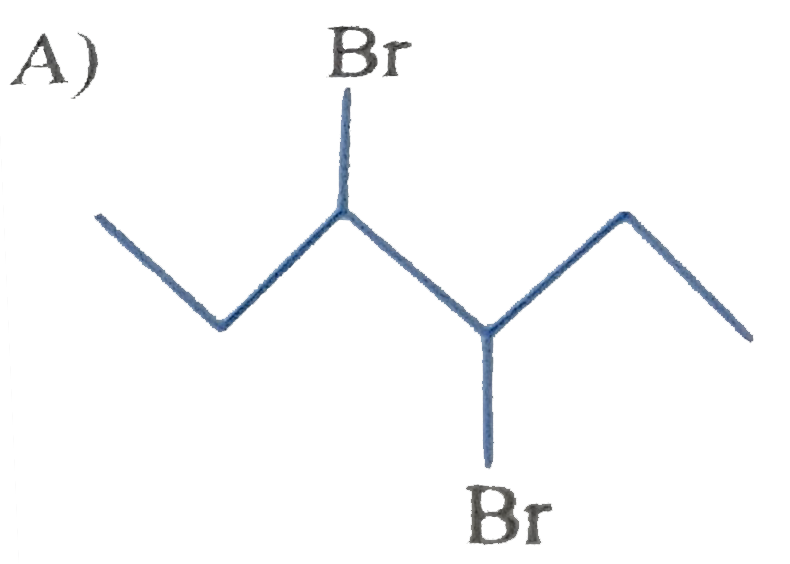
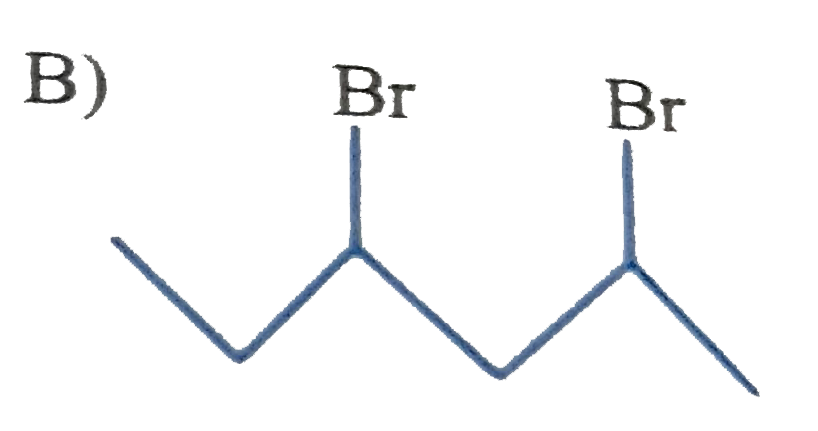
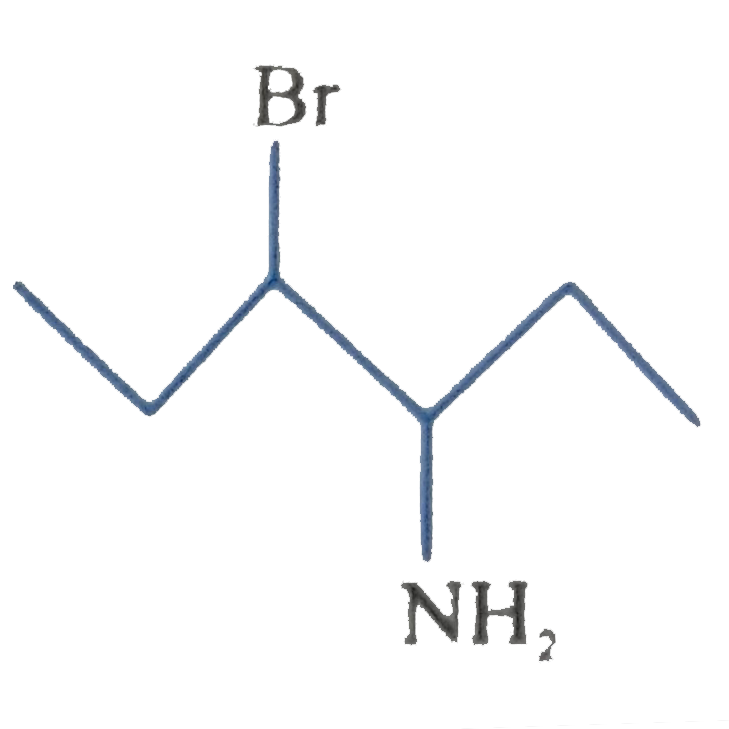
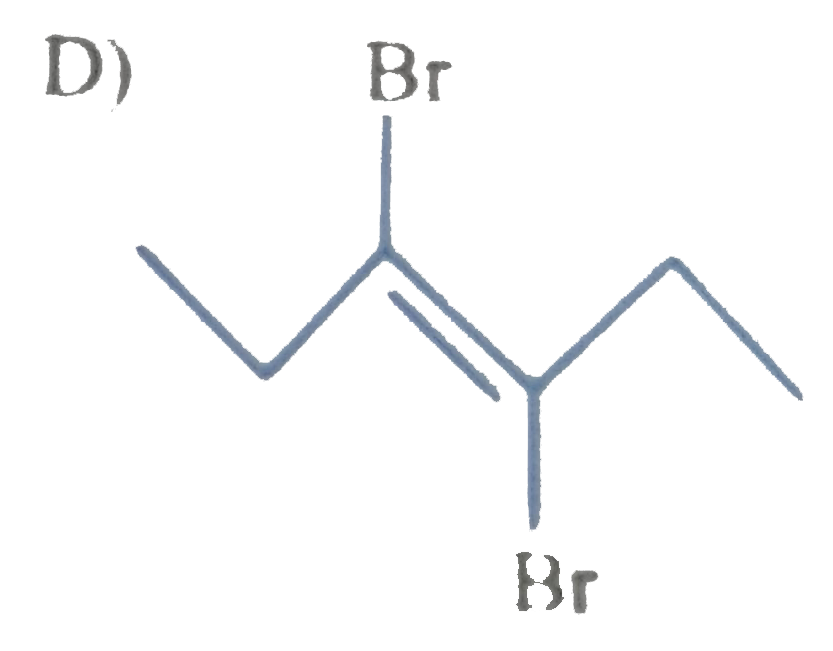
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-ALKYNES-Level-5
- Ph-overset(Cl)overset(|)underset(Cl)underset(|)(C)-CH(3)overset(3NaNH(...
Text Solution
|
- Product (B) is
Text Solution
|
- What is the final product, C, of the following reaction sequence? CH...
Text Solution
|
- Compound X will be
Text Solution
|
- Match the following columns
Text Solution
|
- One mole of 1,2-dibromopropane on treatment with X moles of NaNH(2) fo...
Text Solution
|
- CH(3)-C-=C-CH(3)overset("Cold " KMnO(4))(to)(A) Product (A) is :
Text Solution
|
- In which reaction last production is Ph-C-=CH?
Text Solution
|
- Predict the product of the following reaction sequence: Ethyne overs...
Text Solution
|
- underset((A))(C(4)H(6))underset(Pd//BaSO(4))overset(H(2))tounderset((B...
Text Solution
|
- Which of the following on reductive ozonolysis give only glyoxal?
Text Solution
|
- Which of the following reduces 2-butyne into cis-2-butene.
Text Solution
|
- B is identical when A is
Text Solution
|
- Ammonical silver nitrate reacts with
Text Solution
|
- Which of the following statement are correct:
Text Solution
|
- Which is/are true statement/reactions?
Text Solution
|
- Which gases are poisonous?
Text Solution
|
- In the following sequence of reactions, products (A) to (H) are formed...
Text Solution
|
- In the following sequence of reactions products (A) to (H) are formed....
Text Solution
|
- In the following sequence of reactions products (A) to (H) are formed....
Text Solution
|