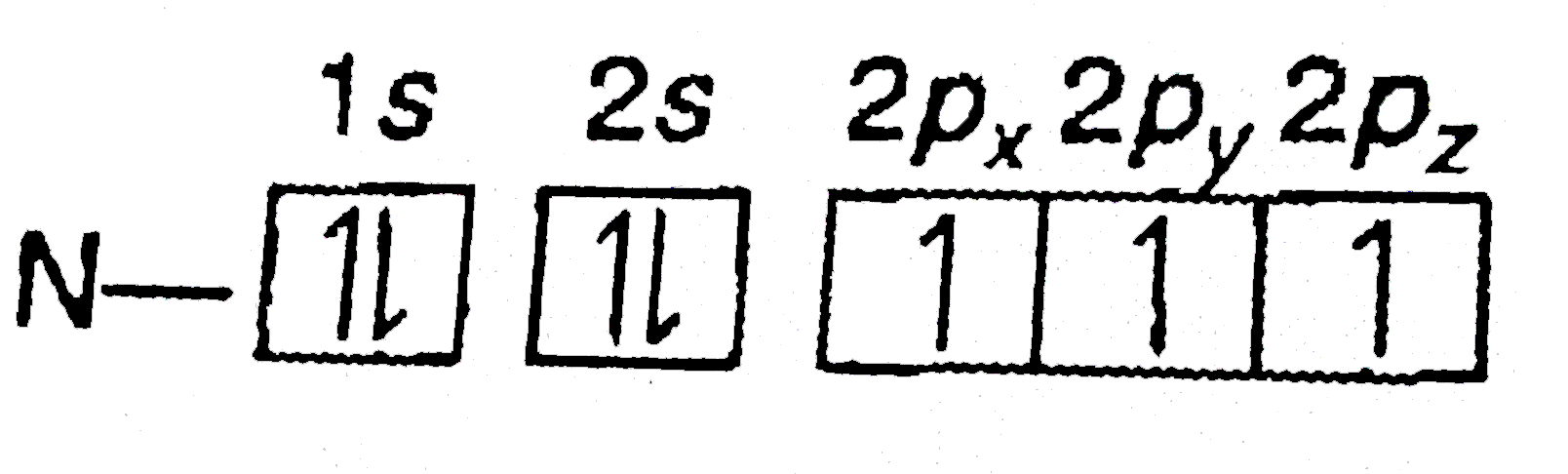A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BITSAT GUIDE-ATOMIC STRUCTURE-Bitsat Archives
- Nitrogen has electronic configuration 1s^(2)2s^(2)2p(x)^(1)2p(y)^(1)2p...
Text Solution
|
- Which of the following relation is incorrect regarding Bohr's theory ?
Text Solution
|
- Bohr theory is applicable to
Text Solution
|
- if the radius of H is 0.53 Å then what will be the radius of .(3)Li^(2...
Text Solution
|
- Which of the following has the largest de - Broglie wavelength given t...
Text Solution
|
- The wave number of a spectral line is 5xx10^(5)m^(-1). The energy corr...
Text Solution
|
- Energy of third orbit of Bohr's atom is
Text Solution
|
- An electronic transition in hydrogen atom result in the formation of H...
Text Solution
|
- The velocities of two particles A and B are 0.05 and 0.02ms^(-1) respe...
Text Solution
|
- Cr has electronic configuration as
Text Solution
|
- The number of waves in an orbit are
Text Solution
|
- The probability of finding the electron in the orbital is
Text Solution
|
- The velocity of electron in first orbit of H-atom as compared to the v...
Text Solution
|