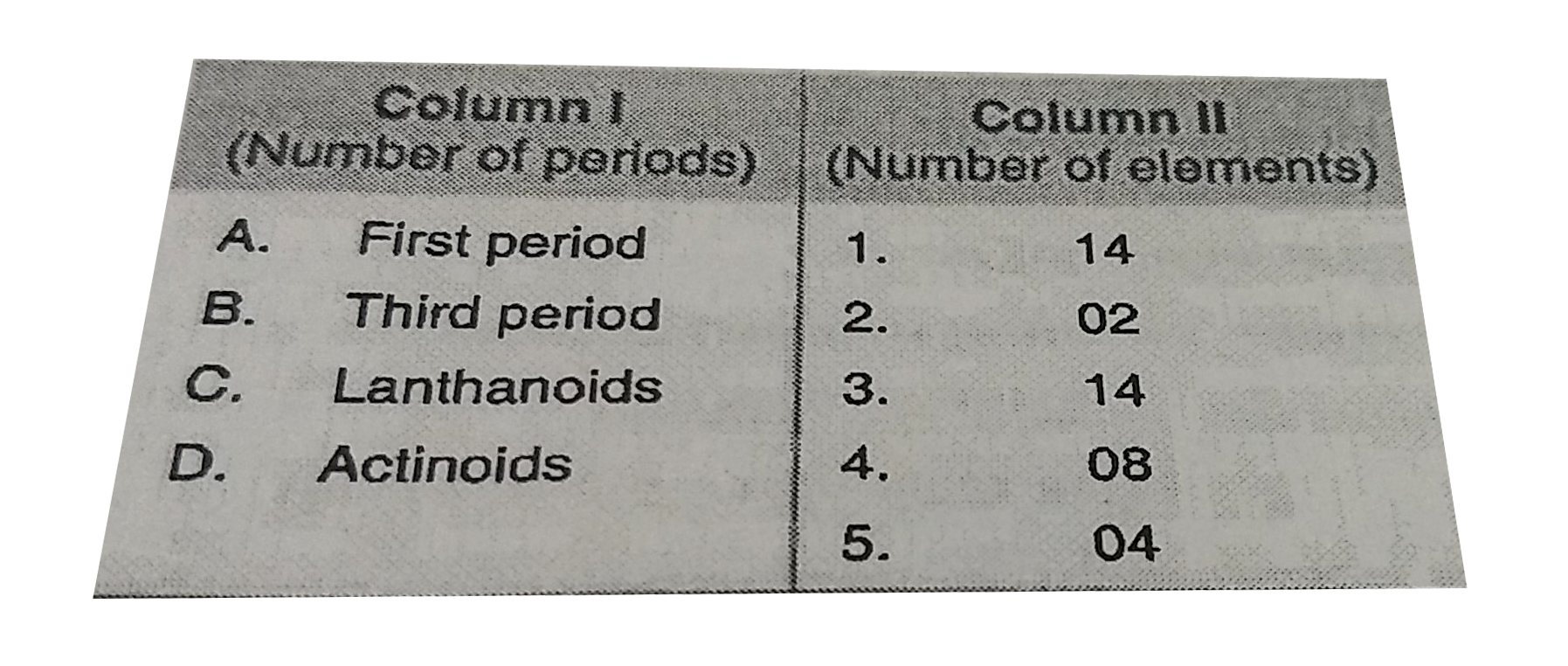
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BITSAT GUIDE-PERIODIC PROPERTIES-Practice Exercise
- The period number in the long form of the periodic table is equal to
Text Solution
|
- Which of the following is incorrect ?
Text Solution
|
- Match the Column I with Column II and select the correct answer by giv...
Text Solution
|
- Observe the following statements, I. The physical and chemical prope...
Text Solution
|
- There are 10 neutrons in the nucleus of the element (Z)M^(19) . It bel...
Text Solution
|
- The third alkaline earth metal ion contains number of electrons and pr...
Text Solution
|
- A metal having electronic configuration 1s^(2), 2s^(2)2p^(6), 3s^(2)...
Text Solution
|
- Which of the following orbitals are in the process of filling in the 6...
Text Solution
|
- The configuration to second excited state of the element is (O) electr...
Text Solution
|
- The element having atomic number 33 lies in the group
Text Solution
|
- General configuration of outermost and penultimate shell is (n - 1) s^...
Text Solution
|
- Generally, the valency of noble gases is
Text Solution
|
- The least stable ion among the following is
Text Solution
|
- The electronic configuration of an element is 1s^(2),2s^(2),2p^(6),3s^...
Text Solution
|
- What will be the IUPAC name of element having Z = 106 ?
Text Solution
|
- The one with the largest ionic size is
Text Solution
|
- Fluorine and neon have atomic radii in angstrom given by
Text Solution
|
- Which of the following alkali metals has smallest size ?
Text Solution
|
- Chloride ion and potassium ion are isoelectronic. Then
Text Solution
|
- Which one of the following is correct increasing order of size ?
Text Solution
|