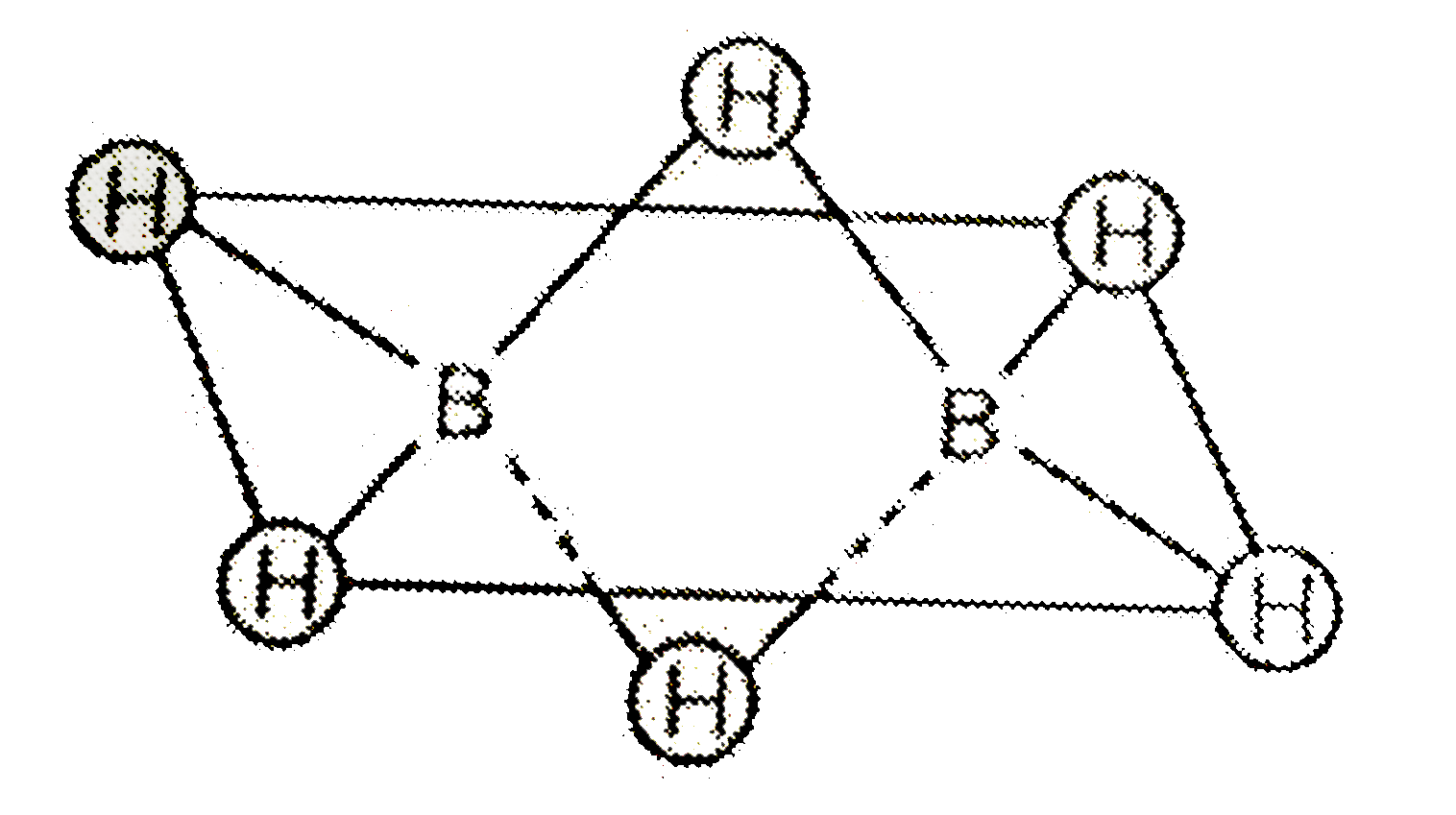
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BITSAT GUIDE-P BLOCK ELEMENTS : GROUP 13,14-Archives
- Which of the following statements are correct regarding diborane? I....
Text Solution
|
- Which of the following staments are incorrect in context of borax?
Text Solution
|
- For the properties mentioned, the correct trend for the different spec...
Text Solution
|
- Which glass has the highest percentage of lead ?
Text Solution
|